Drug Detail:Asclera (Laureth-9 (polidocanol) [ lawr-eth-9 ])
Drug Class: Sclerosing agents
Highlights of Prescribing Information
Asclera (polidocanol) Injection, for intravenous use Initial U.S. Approval: 2010
Indications and Usage for Asclera
Asclera (polidocanol) is a sclerosing agent indicated to sclerose uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower extremity. Asclera has not been studied in varicose veins more than 3mm in diameter. (1)
Asclera Dosage and Administration
For intravenous use only. Solution strength and the volume injected depend on the size and extent of the varicose veins. Extensive varicosities may require multiple treatment sessions. (2)
Spider veins (varicose veins ≤1 mm in diameter): Use Asclera 0.5%. (2)
Reticular veins (varicose veins 1 to 3 mm in diameter): Use Asclera 1%. (2)
Use 0.1 to 0.3 mL for each injection into each varicose vein. The maximum recommended volume per treatment session is 10 mL. (2)
Dosage Forms and Strengths
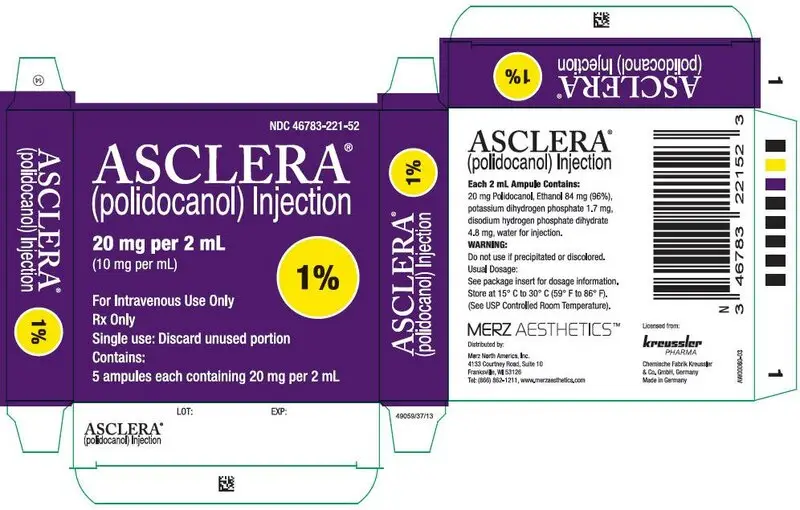
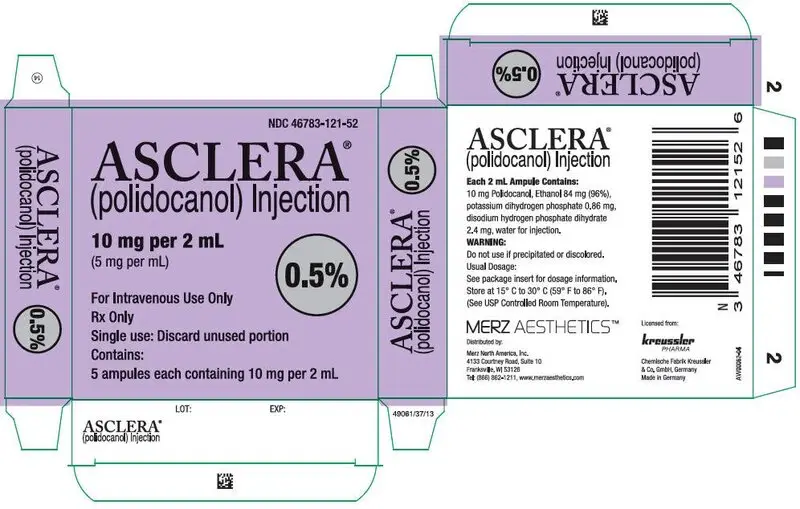
0.5% and 1% solution in 2 mL glass ampules. (3)
Contraindications
Known allergies to polidocanol. (4)
Patients with acute thromboembolic diseases. (4)
Warnings and Precautions
- Be prepared to treat anaphylaxis. (5.1)
- Venous Thrombosis and Pulmonary Embolism. (5.2)
- Arterial Embolism. (5.3)
- Tissue ischemia and necrosis: Do not inject intra-arterially. (5.4)
Adverse Reactions/Side Effects
The most common adverse reactions occurring at least 3% more frequently than on placebo are mild local reactions at the site of injection. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Merz North America, Inc. at 1-844-469-6379 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2019
Full Prescribing Information
1. Indications and Usage for Asclera
Asclera® (polidocanol) is indicated to sclerose uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower extremity. Asclera has not been studied in varicose veins more than 3 mm in diameter.
2. Asclera Dosage and Administration
For intravenous use only. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter is seen or if the contents of the vial are discolored or if the vial is damaged in any way.
For spider veins (varicose veins ≤1 mm in diameter), use Asclera 0.5%. For reticular veins (varicose veins 1 to 3 mm in diameter), use Asclera 1%. Use 0.1 to 0.3 mL per injection and no more than 10 mL per session.
Use a syringe (glass or plastic) with a fine needle (typically, 26- or 30-gauge). Insert the needle tangentially into the vein and inject the solution slowly while the needle is still in the vein. Apply only gentle pressure during injection to prevent vein rupture. After the needle has been removed and the injection site has been covered, apply compression in the form of a stocking or bandage. After the treatment session, encourage the patient to walk for 15 to 20 minutes. Keep the patient under observation to detect any anaphylactic or allergic reaction [see Warnings and Precautions (5.3)].
Maintain compression for 2 to 3 days after treatment of spider veins and for 5 to 7 days for reticular veins. For extensive varicosities, longer compression treatment with compression bandages or a gradient compression stocking of a higher compression class is recommended. Post-treatment compression is necessary to reduce the risk of deep vein thrombosis.
Repeat treatments may be necessary if the extent of the varicose veins requires more than 10 mL. These treatments should be separated by 1 to 2 weeks.
Small intravaricose thrombi that develop may be removed by microthrombectomy.
4. Contraindications
Asclera is contraindicated for patients with known allergy to polidocanol and patients with acute thromboembolic diseases.
5. Warnings and Precautions
5.1 Anaphylaxis
Severe allergic reactions have been reported following polidocanol use, including anaphylactic reactions, some of them fatal. Severe reactions are more frequent with use of larger volumes (> 3 mL). Minimize the dose of polidocanol. Be prepared to treat anaphylaxis appropriately.
Severe adverse local effects, including tissue necrosis, may occur following extravasation; therefore, take care in intravenous needle placement and use the smallest effective volume at each injection.
After the injection session is completed, apply compression with a stocking or bandage, and have the patient walk for 15-20 minutes. Keep the patient under supervision during this period to treat any anaphylactic or allergic reaction [see Dosage and Administration (2)].
5.2 Venous Thrombosis and Pulmonary Embolism
Asclera can cause venous thrombosis and subsequent pulmonary embolism or other thrombotic events. Follow administration instructions closely and monitor for signs of venous thrombosis after treatment. Patients with reduced mobility, history of deep vein thrombosis or pulmonary embolism, or recent (within 3 months) major surgery, prolonged hospitalization or pregnancy are at increased risk for developing thrombosis.
5.3 Arterial Embolism
Stroke, transient ischemic attack, myocardial infarction, and impaired cardiac function have been reported in close temporal relationship with polidocanol administration. These events may be caused by air embolism when using the product foamed with room air (high nitrogen concentration) or thromboembolism. The safety and efficacy of polidocanol foamed with room air has not been established and its use should be avoided.
5.4 Tissue Ischemia and Necrosis
Intra-arterial injection or extravasation of polidocanol can cause severe necrosis, ischemia or gangrene. Take care in intravenous needle placement and use the smallest effective volume at each injection site. After the injection session is completed, apply compression with a stocking or bandage and have patients walk for 15-20 minutes. If intra-arterial injection of polidocanol occurs, consult a vascular surgeon immediately.
8. Use In Specific Populations
11. Asclera Description
Asclera is a sterile, nonpyrogenic, and colorless to faintly greenish-yellow solution of polidocanol for intravenous use as a sclerosing agent.
The active ingredient, polidocanol is a non-ionic detergent, consisting of two components, a polar hydrophilic (dodecyl alcohol) and an apolar hydrophobic (polyethylene oxide) chain. Polidocanol has the following structural formula:
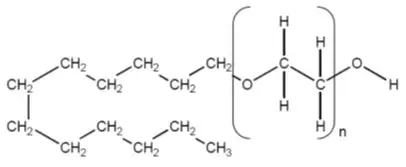
C12H25(OCH2CH2)nOH Polyethylene glycol monododecyl ether Mean extent of polymerization (n) : Approximately 9 Mean molecular weight : Approximately 600
Each mL contains 5 mg (0.5%) or 10 mg (1.0%) polidocanol in water for injection with 5% (v/v) ethanol at pH 6.5-8.0; disodium hydrogen phosphate dihydrate, potassium dihydrogen phosphate are added for pH adjustment.
12. Asclera - Clinical Pharmacology
12.1 Mechanism of Action
The active ingredient of Asclera is polidocanol.
Polidocanol is a sclerosing agent that locally damages the endothelium of blood vessels. When injected intravenously, polidocanol induces endothelial damage. Platelets then aggregate at the site of damage and attach to the venous wall. Eventually, a dense network of platelets, cellular debris, and fibrin occludes the vessel. Finally, the occluded vein is replaced with connective fibrous tissue.
12.2 Pharmacodynamics
Polidocanol has a concentration- and volume-dependent damaging effect on the endothelium of blood vessels.
12.3 Pharmacokinetics
During the major effectiveness study (EASI-trial), scheduled blood samples were taken from a sub-group of 22 patients to measure plasma levels of polidocanol after Asclera treatment of spider and reticular veins. Low systemic blood levels of polidocanol were seen in some patients.
The mean t1/2 of polidocanol in 4 patients with evaluable data receiving 4.5 -18.0 mg was 1.5 h.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate carcinogenic potential have not been conducted with polidocanol. Polidocanol was negative in bacterial reverse mutation assays in Salmonella and E. coli, and in a micronucleus assay conducted in mice. Polidocanol induced numerical chromosomal aberrations in cultured newborn Chinese hamster lung fibroblasts in the absence of metabolic activation.
Polidocanol did not affect reproductive performance (fertility) of rats when administered intermittently at dosages up to 10 mg/kg (approximately equal to the maximum human dose on the basis of body surface area).
14. Clinical Studies
Asclera was evaluated in a multicenter, randomized, double-blind, placebo- and comparator-controlled trial (EASI-study) in patients with spider or reticular varicose veins. A total of 338 patients were treated with Asclera [0.5% for spider veins (n=94), 1% for reticular veins (n=86)], sodium tetradecyl sulfate (STS) 1% (n=105), or placebo (0.9% isotonic saline solution) (n=53) for either spider or reticular veins. Patients were predominately female, ranging in age from 19 to 70 years. All of them received an intravenous injection in the first treatment session; repeat injections were given three and six weeks later if the previous injection was evaluated as unsuccessful (defined as 1, 2 or 3 on a 5-point scale, see below). Patients returned at 12 and 26 weeks after the last injection for final assessments.
The primary effectiveness endpoint was improvement of veins judged by a blinded panel. Digital images of the selected treatment area were taken prior to injection, compared with those taken at 12 weeks post-treatment, and rated on a 5-point scale (1 = worse than before, 2 = same as before, 3 = moderate improvement, 4 = good improvement, 5 = complete treatment success); results are shown in Table 2.
| Treatment Group | Polidocanol (n=155) | STS (n=105) | Placebo (n=53) |
|---|---|---|---|
|
|||
| Digital Photograph Scores at 12 weeks | |||
| Mean ± SD | 4.5* ± 0.7 | 4.5*± 0.7 | 2.2 ± 0.7 |
| Digital Photograph Scores at 26 weeks | |||
| Mean ± SD | 4.5* ± 0.7 | 4.5*± 0.8 | 2.2 ± 0.7 |
The secondary efficacy criterion was the rate of treatment success, pre-defined as a score of 4 or 5 with patients scoring 1, 2, or 3 considered treatment failures; results are shown in Table 3.
| Treatment Success* | Polidocanol (n=155) | STS (n=105) | Placebo (n=53) |
|---|---|---|---|
|
|||
| At 12 weeks (Visit 4) | |||
| Yes | 95%† | 92%† | 8% |
| No | 5% | 8% | 92% |
| Missing | 0.6% | 0% | 0% |
| At 26 weeks (Visit 5) | |||
| Yes | 95%† | 91%† | 6% |
| No | 5% | 9% | 94% |
At 12 and 26 weeks, patients' judgement of the results was assessed by showing them the digital images of their treatment area taken at baseline and asking them to rate their satisfaction with their treatment using a verbal rating scale (1 = very unsatisfied; 2 = somewhat unsatisfied; 3 = slightly satisfied; 4 = satisfied and 5 = very satisfied); results are shown in Table 4.
| Polidocanol (N=155) | STS (N=105) | Placebo (N=53) | |
|---|---|---|---|
|
|||
| Patient satisfaction with treatment after 12 weeks (Visit 4) | |||
| Satisfied or very satisfied | 87%* | 64% | 14% |
| Patient satisfaction with treatment after 26 weeks (Visit 5) | |||
| Satisfied or very satisfied | 84%* | 63% | 16% |
16. How is Asclera supplied
Asclera is supplied in single-use, preservative free ampules in the following packages:
NDC 46783-121-52 Five 0.5% ampules (2 mL)
NDC 46783-221-52 Five 1.0% ampules (2 mL)
Each ampule is intended for immediate use in a single patient. Each unopened ampule is stable up to three years.
17. Patient Counseling Information
Advise the patient to wear compression stockings or support hose on the treated legs continuously for 2 to 3 days and for 2 to 3 weeks during the daytime. Compression stockings or support hose should be thigh or knee high depending upon the area treated in order to provide adequate coverage.
Advise the patient to walk for 15 to 20 minutes immediately after the procedure and daily for the next few days.
For two to three days following treatment, advise the patient to avoid heavy exercise, sunbathing, long plane flights, and hot baths or sauna.
Distributed by:
Merz North America, Inc.
6501 Six Forks Road
Raleigh, NC 27615
Manufactured by:
Chemische Fabrik Kreussler & Co. GmbH 65203 Wiesbaden GERMANY
Asclera is a registered trademark of Chemische Fabrik Kreussler & Co. GmbH, 65203 Wiesbaden, GERMANY
| ASCLERA
polidocanol injection, solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ASCLERA
polidocanol injection, solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Merz North America, Inc (028147846) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Chemische Fabrik Kreussler & Co. GmbH | 315568337 | MANUFACTURE(46783-221, 46783-121) | |