Drug Detail:Asenapine (monograph) (Saphris)
Drug Class:
Highlights of Prescribing Information
ASENAPINE sublingual tablets
Initial U.S. Approval: 2009
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Asenapine sublingual tablets are not approved for the treatment of patients with dementia-related psychosis. (5.1, 5.2)
Recent Major Changes
| Indications and Usage (1) | 01/2017 |
| Dosage and Administration (2.3) | 01/2017 |
| Contraindications (4) | 01/2017 |
| Warnings and Precautions (5.1, 5.2, 5.3, 5.4, 5.5, 5.7, 5.9, 5.10, 5.11, 5.12, 5.13, 5.14, 5.15) | 01/2017 |
| Warnings and Precautions (5.8) | 02/2017 |
Indications and Usage for Asenapine Tablets
Asenapine sublingual tablets are an atypical antipsychotic indicated for (1):
- Schizophrenia in adults
- Bipolar I disorder
- Acute monotherapy treatment of manic or mixed episodes, in adults and pediatric patients 10 to 17 years of age
- Adjunctive treatment to lithium or valproate in adults
- Maintenance monotherapy treatment in adults
Asenapine Tablets Dosage and Administration
| Starting Dose | Recommended Dose | Maximum Dose | |
|---|---|---|---|
| Schizophrenia – acute treatment in adults (2.2) | 5 mg sublingually twice daily | 5 mg sublingually twice daily | 10 mg sublingually twice daily |
| Schizophrenia – maintenance treatment in adults (2.2) | 5 mg sublingually twice daily | 5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
| Bipolar mania-adults: acute and maintenance monotherapy (2.3) | 5-10 mg sublingually twice daily | 5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
| Bipolar mania – pediatric patients (10 to 17 years): monotherapy (2.3) | 2.5 mg sublingually twice daily | 2.5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
| Bipolar mania – adults: as an adjunct to lithium or valproate (2.3) | 5 mg sublingually twice daily | 5-10 mg sublingually twice daily | 10 mg sublingually twice daily |
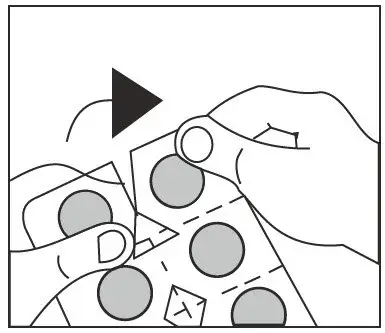
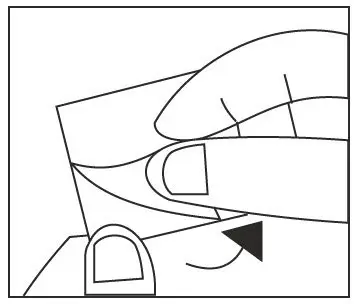
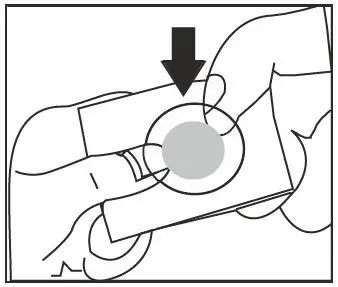
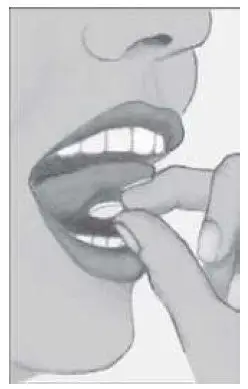
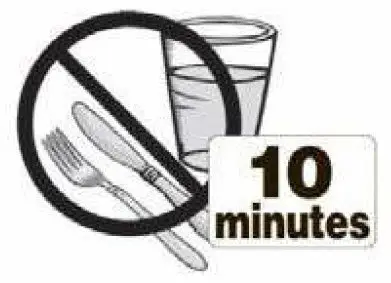
- Do not swallow tablet. Asenapine sublingual tablets should be placed under the tongue and left to dissolve completely. The tablet will dissolve in saliva within seconds. Eating and drinking should be avoided for 10 minutes after administration. (2.1, 17)
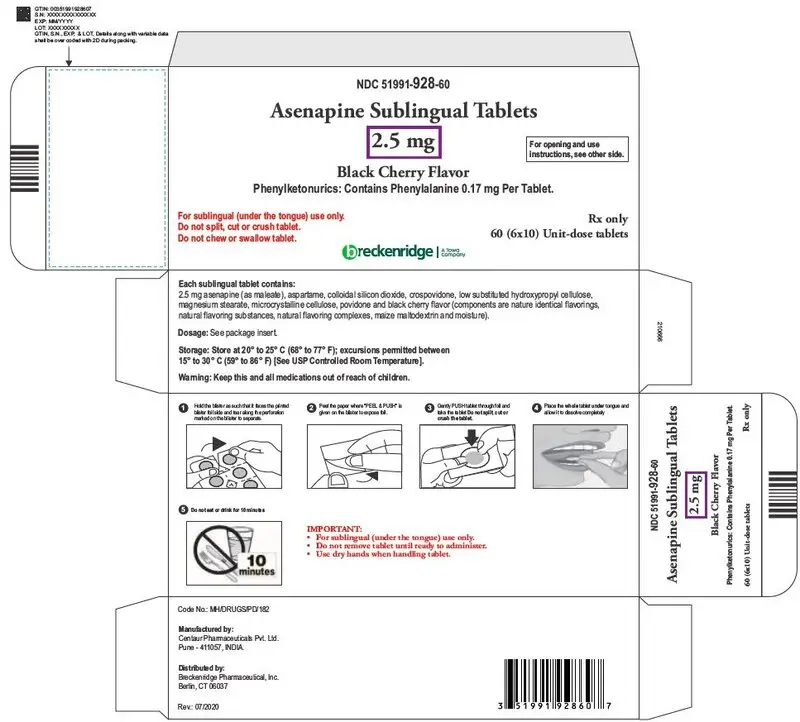
Dosage Forms and Strengths
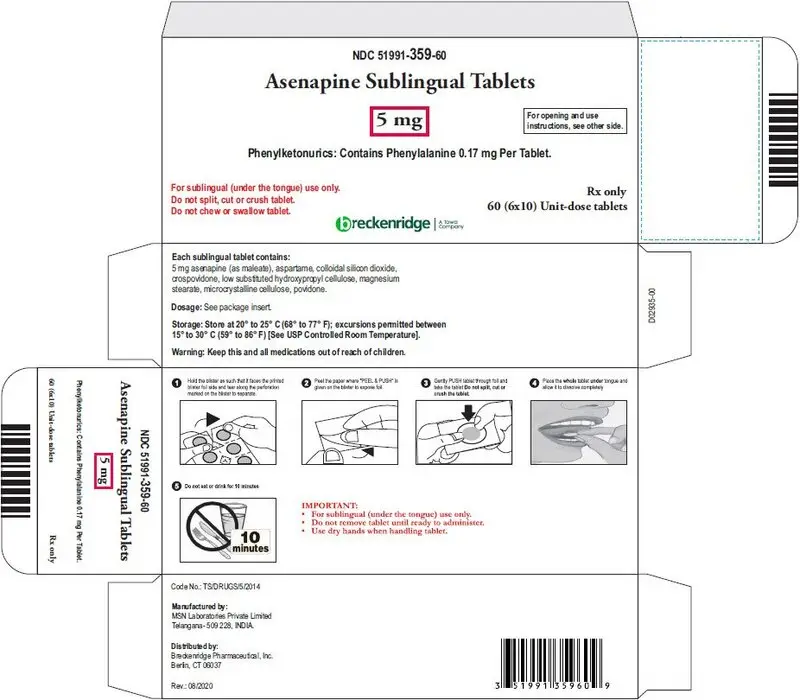
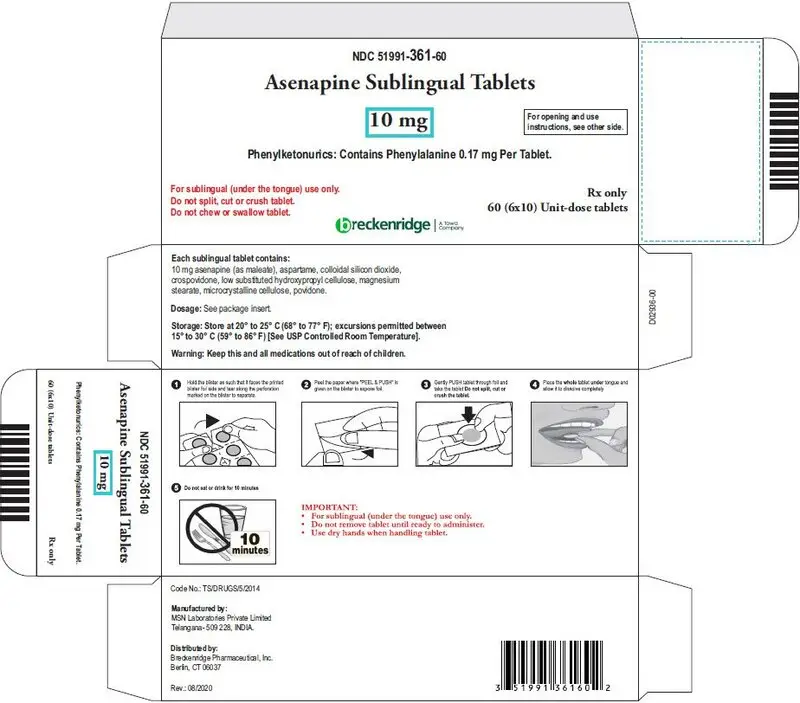
Sublingual tablets: 5 mg and 10 mg (3)
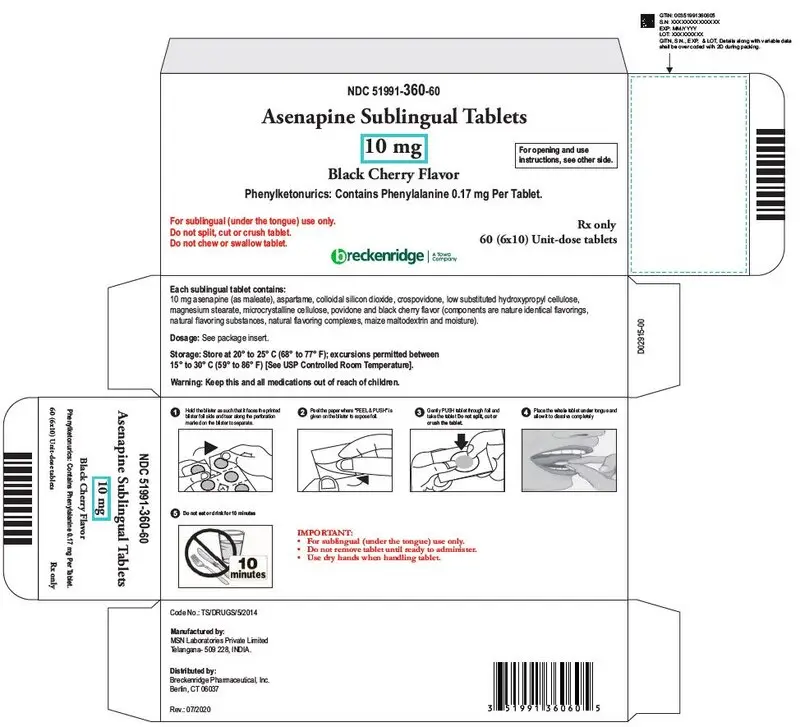
Sublingual tablets, black cherry flavor: 2.5 mg, 5 mg and 10 mg (3)
Contraindications
- Severe hepatic impairment (Child-Pugh C). (8.7, 12.3)
- Known hypersensitivity to asenapine sublingual tablets, or to any components in the formulation. (4, 5.6, 17)
Warnings and Precautions
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack). (5.2)
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring. (5.3)
- Tardive Dyskinesia: Discontinue if clinically appropriate. (5.4)
- Metabolic Changes: Monitor for hyperglycemia/diabetes mellitus, dyslipidemia, and weight gain. (5.5)
- Orthostatic Hypotension: Monitor heart rate and blood pressure and warn patients with known cardiovascular or cerebrovascular disease, and risk of dehydration or syncope. (5.7)
- Leukopenia, Neutropenia, and Agranulocytosis: Perform complete blood counts (CBC) in patients with pre-existing low white blood cell count (WBC) or history of leukopenia or neutropenia. Consider discontinuing asenapine sublingual tablets if a clinically significant decline in WBC occurs in absence of other causative factors. (5.9)
- QT Prolongation: Increases in QT interval; avoid use with drugs that also increase the QT interval and in patients with risk factors for prolonged QT interval. (5.10)
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold. (5.12)
- Potential for Cognitive and Motor Impairment: Use caution when operating machinery. (5.13)
Adverse Reactions/Side Effects
The most commonly observed adverse reactions (incidence ≥5% and at least twice that for placebo) were (6.1):
- Schizophrenia Adults: akathisia, oral hypoesthesia, somnolence.
- Bipolar I Disorder Adults (Monotherapy): somnolence, oral hypoesthesia, dizziness, extrapyramidal symptoms (excluding akathisia) and akathisia.
- Bipolar I Disorder Pediatric Patients (Monotherapy): somnolence, dizziness, dysgeusia, oral paresthesia, nausea, increased appetite, fatigue, increased weight.
- Bipolar I Disorder Adults (Adjunctive): somnolence, oral hypoesthesia.
To report SUSPECTED ADVERSE REACTIONS, contact Breckenridge Pharmaceutical, Inc. at 1-800-367-3395 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Antihypertensive Drugs: Asenapine sublingual tablets may cause hypotension. (5.7, 7.1, 12.3)
- Paroxetine (CYP2D6 substrate and inhibitor): Reduce paroxetine by half when used in combination with asenapine sublingual tablets. (7.1, 12.3)
Use In Specific Populations
- Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. (8.1)
- Pediatric Use: Safety and efficacy in the treatment of bipolar I disorder in patients less than 10 years of age, and patients with schizophrenia ages less than 12 years have not been evaluated. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2021
Related/similar drugs
Vraylar, Abilify Maintena, Caplyta, quetiapine, Abilify, lamotrigine, SeroquelFull Prescribing Information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Asenapine sublingual tablets are not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1, 5.2)].
1. Indications and Usage for Asenapine Tablets
Asenapine sublingual tablets are indicated for:
- Schizophrenia in adults [see Clinical Studies (14.1)]
-
Bipolar I disorder [see Clinical Studies (14.2)]
- Acute monotherapy of manic or mixed episodes, in adults and pediatric patients 10 to 17 years of age
- Adjunctive treatment to lithium or valproate in adults
- Maintenance monotherapy treatment in adults
2. Asenapine Tablets Dosage and Administration
2.1 Administration Instructions
Asenapine is a sublingual tablet. To ensure optimal absorption, patients should be instructed to place the tablet under the tongue and allow it to dissolve completely. The tablet will dissolve in saliva within seconds. Asenapine sublingual tablets should not be split, crushed, chewed, or swallowed [see Clinical Pharmacology (12.3)]. Patients should be instructed to not eat or drink for 10 minutes after administration [see Clinical Pharmacology (12.3)].
2.2 Schizophrenia
The recommended dose of asenapine sublingual tablets is 5 mg given twice daily. In short-term controlled trials, there was no suggestion of added benefit with a 10 mg twice daily dose, but there was a clear increase in certain adverse reactions. If tolerated, daily dosage can be increased to 10 mg twice daily after one week. The safety of doses above 10 mg twice daily has not been evaluated in clinical studies [see Clinical Studies (14.1)].
3. Dosage Forms and Strengths
- Asenapine sublingual 5 mg tablets are round, white, uncoated tablets debossed "B" on one side and "1" on the other side.
- Asenapine sublingual 10 mg tablets are round, white, uncoated tablets debossed "B" on one side and "2" on the other side.
- Asenapine sublingual 2.5 mg tablets, black cherry flavor, are white colored, round shaped, uncoated tablets debossed with "B" on one side and "5" on the other side
- Asenapine sublingual 5 mg tablets, black cherry flavor, are round, white, uncoated tablets debossed "B" on one side and "3" on the other side.
- Asenapine sublingual 10 mg tablets, black cherry flavor, are round, white, uncoated tablets debossed "B" on one side and "4" on the other side.
4. Contraindications
Asenapine sublingual tablets are contraindicated in patients with:
- Severe hepatic impairment (Child-Pugh C) [see Specific Populations (8.7), Clinical Pharmacology (12.3)].
- A history of hypersensitivity reactions to asenapine. Reactions have included anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash [see Warnings and Precautions (5.6), Adverse Reactions (6)].
5. Warnings and Precautions
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Asenapine sublingual tablets are not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions (5.2)].
5.2 Cerebrovascular Adverse Events, Including Stroke, In Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials in elderly subjects with dementia, patients randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. Asenapine sublingual tablets are not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning, Warnings and Precautions (5.1)].
5.3 Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, delirium, and autonomic instability. Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. If NMS is suspected, immediately discontinue asenapine sublingual tablets and provide intensive symptomatic treatment and monitoring.
5.4 Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs, including asenapine sublingual tablets. The risk appears to be highest among the elderly, especially elderly women, but it is not possible to predict which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of tardive dyskinesia and the likelihood that it will become irreversible increase with the duration of treatment and the cumulative dose. The syndrome can develop after a relatively brief treatment period, even at low doses. It may also occur after discontinuation of treatment.
There is no known treatment for tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of tardive dyskinesia is unknown.
Given these considerations, asenapine sublingual tablets should be prescribed in a manner most likely to reduce the risk of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients: 1) who suffer from a chronic illness that is known to respond to antipsychotic drugs; and 2) for whom alternative, effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. Periodically reassess the need for continued treatment.
If signs and symptoms of TD appear in a patient on asenapine sublingual tablets, drug discontinuation should be considered. However, some patients may require treatment with asenapine sublingual tablets despite the presence of the syndrome.
5.5 Metabolic Changes
Atypical antipsychotic drugs, including asenapine sublingual tablets, have caused metabolic changes, including hyperglycemia, diabetes mellitus, dyslipidemia, and body weight gain. Although all of the drugs in the class to date have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Dyslipidemia
Atypical antipsychotics cause adverse alterations in lipids. Before or soon after initiation of antipsychotic medication, obtain a fasting lipid profile at baseline and monitor periodically during treatment.
Adult Patients: Pooled data from the short-term, placebo-controlled schizophrenia and bipolar mania trials are presented in Table 3.
| Schizophrenia (6-weeks) | Bipolar I Disorder (3-weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Asenapine sublingual tablets | Placebo | Asenapine sublingual tablets | |||||
| 5 mg twice daily | 10 mg twice daily | 5 or 10 mg twice daily* | 5 mg twice daily | 10 mg twice daily | 5 or 10 mg twice daily† | |||
|
||||||||
| Mean Change from Baseline (mg/dL) | ||||||||
| Total cholesterol (N‡) | -2.2 (351) | -2.4 (258) | 3.3 (199) | 0.4 (539) | -1.6 (278) | -1.6 (108) | -4.7 (95) | -0.5 (525) |
| LDL (N‡) | 0.1 (285) | -0.2 (195) | 2.6 (195) | 1.3 (465) | 1.4 (271) | -2.5 (101) | -4.1 (94) | -0.3 (499) |
| HDL (N‡) | 0.5 (290) | 0.4 (199) | 1.0 (199) | 0.5 (480) | 0.2 (278) | 0.1 (108) | 0.7 (95) | 0.7 (525) |
| Fasting triglycerides (N‡) | -7.6 (233) | -1.9 (159) | 0.1 (154) | 3.8 (380) | -16.9 (222) | 3.9 (89) | -8.5 (85) | -3.0 (411) |
| Proportion of Patients with Shifts from Baseline to Endpoint | ||||||||
| Total cholesterol Normal to High <200 to ≥240 (mg/dL) (n/N‡) | 1.3% (3/225) | 0.6% (1/161) | 2.2% (3/134) | 1.7% (6/343) | 1.2 (2/174) | 3.0 (2/66) | 0 (0/63) | 2.1 (7/333) |
| LDL Normal to High <100 to ≥160 (mg/dL) (n/N‡) | 1.7% (2/117) | 0.0% (0/80) | 1.2% (1/86) | 1.0% (2/196) | 1.9 (2/108) | 2.4 (1/41) | 0 (0/41) | 0.5 (1/223) |
| HDL Normal to Low ≥40 to <40 (mg/dL) (n/N‡) | 10.7% (21/196) | 13.3% (18/135) | 14.7% (20/136) | 14.0% (45/322) | 7.4 (16/215) | 4.1 (4/97) | 5.1 (4/78) | 7.0 (29/417) |
| Fasting triglycerides Normal to High <150 to ≥200 (mg/dL) (n/N‡) | 2.4% (4/167) | 7.0% (8/115) | 8.3% (9/108) | 7.7% (20/260) | 4.6 (7/153) | 8.2 (5/61) | 1.6 (1/64) | 6.2 (17/273) |
In short-term schizophrenia trials, the proportion of patients with total cholesterol elevations ≥240 mg/dL (at Endpoint) was 8.3% for asenapine sublingual tablets-treated patients versus 7% for placebo-treated patients. The proportion of patients with elevations in triglycerides ≥200 mg/dL (at Endpoint) was 13.2% for asenapine sublingual tablets-treated patients versus 10.5% for placebo-treated patients. In short-term, placebo-controlled bipolar mania trials, the proportion of patients with total cholesterol elevations ≥240 mg/dL (at Endpoint) was 7.8% for asenapine sublingual tablets-treated patients versus 7.9% for placebo-treated patients. The proportion of patients with elevations in triglycerides ≥200 mg/dL (at Endpoint) was 13.1% for asenapine sublingual tablets-treated patients versus 8.6% for placebo-treated patients.
Pediatric Patients: Data from the short-term, placebo-controlled bipolar mania trial are presented in Table 4
| Bipolar I Disorder (3-weeks) | ||||
|---|---|---|---|---|
| Placebo | Asenapine sublingual tablets 2.5 mg twice daily | Asenapine sublingual tablets 5 mg twice daily | Asenapine sublingual tablets 10 mg twice daily |
|
|
||||
| Mean Change from Baseline (mg/dL) | ||||
| Total fasting cholesterol (N*) | -2.3 (57) | 3.7 (50) | 7.2 (57) | 9.3 (52) |
| Fasting LDL (N*) | -2.5 (57) | -0.2 (50) | 3.0 (57) | 4.9 (51) |
| Fasting HDL (N*) | 1.6 (57) | 2.3 (50) | 1.5 (57) | 1.7 (52) |
| Fasting triglycerides (N*) | -6.6 (57) | 8.7 (50) | 13.4 (57) | 14.7 (52) |
| Proportion of Subjects with Shifts from Baseline to Endpoint | ||||
| Total fasting cholesterol Normal to High <170 to >=200 (mg/dL) (n/N*) | 1.8% (1/57) | 0% (0/50) | 1.8% (1/57) | 0% (0/52) |
| Fasting LDL Normal to High <110 to >=130 (n/N*) | 1.8% (1/57) | 2.0% (1/50) | 1.8% (1/57) | 0% (0/51) |
| Fasting HDL Normal to Low ≥40 to <40 (mg/dL) (n/N*) | 3.5% (2/57) | 6.0% (3/50) | 3.5% (2/57) | 9.6% (5/52) |
| Fasting triglycerides Normal to High <150 to ≥200 (mg/dL) (n/N*) | 0% (0/57) | 4.0% (2/50) | 3.5% (2/57) | 1.9% (1/52) |
Weight Gain
Weight gain has been observed in patients treated with atypical antipsychotics, including asenapine sublingual tabelts. Monitor weight at baseline and frequently thereafter.
Adult Patients: Pooled data on mean changes in body weight and the proportion of subjects meeting a weight gain criterion of ≥7% of body weight from the short-term, placebo-controlled schizophrenia and bipolar mania trials are presented in Table 5.
| Schizophrenia (6-weeks) | Bipolar I Disorder (3-weeks) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Asenapine sublingual tablets | Placebo | Asenapine sublingual tablets | |||||
| 5 mg twice daily | 10 mg twice daily | 5 or 10 mg twice daily* | 5 mg twice daily | 10 mg twice daily | 5 or 10 mg twice daily† | |||
|
||||||||
| Change from Baseline (kg) (N‡) | 0.0 (348) | 1.0 (251) | 0.9 (200) | 1.1 (532) | 0.2 (288) | 1.4 (110) | 1.3 (98) | 1.3 (544) |
| Proportion of Patients with a ≥ 7% Increase in Body weight | ||||||||
| % with ≥7% increase in body weight | 1.6% | 4.4% | 4.8%. | 4.9% | 0.4% | 6.4% | 1.0% | 5.5% |
5.6 Hypersensitivity Reactions
Hypersensitivity reactions have been observed in patients treated with asenapine sublingual tablets. In several cases, these reactions occurred after the first dose. These hypersensitivity reactions included: anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash.
5.7 Orthostatic Hypotension, Syncope, and Other Hemodynamic Effects
Atypical antipsychotics cause orthostatic hypotension and syncope. Generally, the risk is greatest during initial dose titration and when increasing the dose. In short-term schizophrenia adult trials, syncope was reported in 0.2% (1/572) of patients treated with therapeutic doses (5 mg or 10 mg twice daily) of asenapine sublingual tablets, compared to 0.3% (1/378) of patients treated with placebo. In short-term bipolar mania adult trials, syncope was reported in 0.2% (1/620) of patients treated with therapeutic doses (5 mg or 10 mg twice daily) of asenapine sublingual tablets, compared to 0% (0/329) of patients treated with placebo. During adult pre-marketing clinical trials with asenapine sublingual tablets, including long-term trials without comparison to placebo, syncope was reported in 0.6% (11/1,953) of patients treated with asenapine sublingual tablets. In a 3-week, bipolar mania pediatric trial, syncope was reported in 1% (1/104) of patients treated with asenapine sublingual tablets 2.5 mg twice daily, 1% (1/99) of patients treated with asenapine 5 mg twice daily, and 0% (0/99) for patients treated with asenapine sublingual tablets 10 mg twice daily compared to 0% (0/101) for patients treated with placebo.
Orthostatic vital signs should be monitored in patients who are vulnerable to hypotension (elderly patients, patients with dehydration, hypovolemia, concomitant treatment with antihypertensive medications, patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure, or conduction abnormalities), and patients with cerebrovascular disease. Asenapine sublingual tablets should be used cautiously when treating patients who receive treatment with other drugs that can induce hypotension, bradycardia, respiratory or central nervous system depression [see Drug Interactions (7.1)]. Monitoring of orthostatic vital signs should be considered in all such patients, and a dose reduction should be considered if hypotension occurs.
5.8 Falls
Asenapine sublingual tablets may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.9 Leukopenia, Neutropenia, and Agranulocytosis
In clinical trial and postmarketing experience, leukopenia and neutropenia have been reported temporally related to antipsychotic agents, including asenapine sublingual tablets. Agranulocytosis (including fatal cases) has been reported with other agents in the class.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) or absolute neutrophil count (ANC) and history of drug induced leukopenia/neutropenia. In patients with a pre-existing low WBC or ANC or a history of drug-induced leukopenia or neutropenia, perform a complete blood count (CBC) during the first few months of therapy. In such patients, consider discontinuation of asenapine sublingual tablets at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue asenapine sublingual tablets in patients with severe neutropenia (absolute neutrophil count <1000/mm3) and follow their WBC until recovery.
5.10 QT Prolongation
The effects of asenapine sublingual tablets on the QT/QTc interval were evaluated in a dedicated adult QT study. This trial involved asenapine sublingual tablets doses of 5 mg, 10 mg, 15 mg, and 20 mg twice daily, and placebo, and was conducted in 151 clinically stable patients with schizophrenia, with electrocardiographic assessments throughout the dosing interval at baseline and steady state. At these doses, asenapine sublingual tablets was associated with increases in QTc interval ranging from 2 to 5 msec compared to placebo. No patients treated with asenapine sublingual tablets experienced QTc increases ≥60 msec from baseline measurements, nor did any patient experience a QTc of ≥500 msec.
Electrocardiogram (ECG) measurements were taken at various time points during the asenapine sublingual tablets clinical trial program (5 mg or 10 mg twice daily doses). Post-baseline QT prolongations exceeding 500 msec were reported at comparable rates for asenapine sublingual tablets and placebo in these short-term trials. There were no reports of Torsade de Pointes or any other adverse reactions associated with delayed ventricular repolarization.
The use of asenapine sublingual tablets should be avoided in combination with other drugs known to prolong QTc including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and antibiotics (e.g., gatifloxacin, moxifloxacin). Asenapine sublingual tablets should also be avoided in patients with a history of cardiac arrhythmias and in other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including bradycardia; hypokalemia or hypomagnesemia; and presence of congenital prolongation of the QT interval.
5.11 Hyperprolactinemia
Like other drugs that antagonize dopamine D2 receptors, asenapine sublingual tablets can elevate prolactin levels, and the elevation can persist during chronic administration. Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects. In asenapine sublingual tablets adult pre-marketing clinical trials, the incidences of adverse events related to abnormal prolactin levels were 0.4% versus 0% for placebo. In a 3-week, bipolar mania pediatric trial, the incidence of adverse events related to abnormal prolactin levels were 0% in the asenapine sublingual tablets 2.5 mg twice daily treatment group, 2% in the asenapine sublingual tablets 5 mg twice daily treatment group, and 1% in the asenapine sublingual tablets 10 mg twice daily treatment group versus to 1% for patients treated with placebo [see Adverse Reactions (6.1)].
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of these drugs is considered in a patient with previously-detected breast cancer. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive.
5.12 Seizures
Seizures were reported in 0% and 0.3% (0/572,1/379) of adult patients treated with doses of 5 mg and 10 mg twice daily of asenapine sublingual tablets, respectively, compared to 0% (0/503, 0/203) of patients treated with placebo in pre-marketing short-term schizophrenia and bipolar mania trials, respectively. During adult pre-marketing clinical trials with asenapine sublingual tablets, including long-term trials without comparison to placebo, seizures were reported in 0.3% (5/1,953) of patients treated with asenapine sublingual tablets. There were no reports of seizures in pediatric patients treated with asenapine sublingual tablets in a 3-week-term, bipolar mania trial.
As with other antipsychotic drugs, asenapine sublingual tablets should be used with caution in patients with a history of seizures or with conditions that potentially lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in patients 65 years or older.
5.13 Potential for Cognitive and Motor Impairment
Somnolence was reported in patients treated with asenapine sublingual tablets. It was usually transient with the highest incidence reported during the first week of treatment. In short-term, fixed-dose, placebo-controlled schizophrenia adult trials, somnolence was reported in 15% (41/274) of patients on asenapine sublingual tablets 5 mg twice daily and in 13% (26/208) of patients on asenapine sublingual tablets 10 mg twice daily compared to 7% (26/378) of placebo patients. In short-term, placebo-controlled bipolar mania adult trials of therapeutic doses (5-10 mg twice daily), somnolence was reported in 23% (145/620) of patients on asenapine sublingual tablets compared to 5% (18/329) of placebo patients. In the 3-week fixed-dose study, somnolence occurred at a lower rate in the 5mg twice daily dose 20% (24/122) versus the 10mg twice daily dose 26% (31/119) compared to 4% (5/126) in placebo patients. During adult pre-marketing clinical trials with asenapine sublingual tablets, including long-term trials without comparison to placebo, somnolence was reported in 18% (358/1,953) of patients treated with asenapine sublingual tablets. Somnolence led to discontinuation in 0.6% (12/1,953) of patients in short-term, placebo-controlled trials.
In a 3-week, placebo-controlled, bipolar I pediatric trial, the incidence of somnolence (including sedation and hypersomnia) for placebo, asenapine sublingual tablets 2.5 mg twice daily, 5 mg twice daily, and 10 mg twice daily, was 12% (12/101), 46% (48/104), 53% (52/99), and 49% (49/99), respectively. Somnolence led to discontinuation in 0%, 3%, 1%, and 2% of patients treated with placebo, and asenapine sublingual tablets 2.5 mg twice daily, 5 mg twice daily, and 10 mg twice daily, respectively.
Patients should be cautioned about operating hazardous machinery, including motor vehicles, until they are reasonably certain that asenapine sublingual tablets therapy does not affect them adversely.
5.14 Body Temperature Regulation
Atypical antipsychotics may disrupt the body's ability to reduce core body temperature. In the pre-marketing short-term placebo-controlled trials for both schizophrenia and acute bipolar I disorder, the incidence of adverse reactions suggestive of body temperature increases was low (≤1%) and comparable to placebo (0%). During pre-marketing clinical trials with asenapine sublingual tablets, including long-term trials without comparison to placebo, the incidence of adverse reactions suggestive of body temperature increases (pyrexia and feeling hot) was ≤1%.
Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic medications may contribute to an elevation in core body temperature; use asenapine sublingual tablets with caution in patient who may experience these conditions.
5.15 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Dysphagia has been reported with asenapine sublingual tablets. Asenapine sublingual tablets and other antipsychotic drugs should be used cautiously in patients at risk for aspiration.
5.16 Risks in Patients with Phenylketonuria
Phenylketonurics: Contains Phenylalanine 0.17 mg per tablet.
Phenylalanine can be harmful to patients with phenylketonuria (PKU). Asenapine sublingual tablets contain phenylalanine, a component of aspartame. Each 2.5 mg, 5 mg and 10 mg tablets contains 0.17 mg of phenylalanine. Before prescribing asenapine sublingual tablets in a patient with PKU, consider the combined daily amount of phenylalanine from all sources, including asenapine sublingual tablets.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Use in Elderly Patients with Dementia-Related Psychosis [see Boxed Warning and Warnings and Precautions (5.1 and 5.2)]
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.3)]
- Tardive Dyskinesia [see Warnings and Precautions (5.4)]
- Metabolic Changes [see Warnings and Precautions (5.5)]
- Hypersensitivity Reactions [see Contraindications, Warnings and Precautions (5.6)]
- Orthostatic Hypotension, Syncope, and other Hemodynamic Effects [see Warnings and Precautions (5.7)]
- Falls [see Warnings and Precautions (5.8)]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.9)]
- QT Interval Prolongation [see Warnings and Precautions (5.10)]
- Hyperprolactinemia [see Warnings and Precautions (5.11)]
- Seizures [see Warnings and Precautions (5.12)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.13)]
- Body Temperature Regulation [see Warnings and Precautions (5.14)]
- Dysphagia [see Warnings and Precautions (5.15)]
The most common adverse reactions (≥5% and at least twice the rate of placebo) reported with acute treatment in adults with schizophrenia were akathisia, oral hypoesthesia, and somnolence. The safety profile of asenapine sublingual tablets in the maintenance treatment of schizophrenia in adults was similar to that seen with acute treatment.
The most common adverse reactions (≥5% and at least twice the rate of placebo) reported with acute monotherapy treatment of manic or mixed episodes associated with bipolar I disorder in adults were somnolence, oral hypoesthesia dizziness, extrapyramidal symptoms (excluding akathisia) and akathisia; and during the adjunctive therapy trial in bipolar I disorder in adults were somnolence and oral hypoesthesia. The rates were lower at the 5mg twice daily dose than the 10mg twice daily dose for all of these most common adverse reactions. The safety profile of asenapine sublingual tablets in the maintenance treatment of manic or mixed episodes associated with bipolar I disorder in adults was similar to that seen with acute treatment.
The adult information below is derived from a clinical trial database for asenapine sublingual tablets consisting of over 5,355 patients and/or healthy subjects exposed to one or more sublingual doses of asenapine sublingual tablets. A total of 1,427 asenapine-treated patients were treated for at least 24 weeks and 785 asenapine sublingual tablets-treated patients had at least 52 weeks of exposure at therapeutic doses.
In a 3-week monotherapy trial, the most common adverse reactions (≥5% and at least twice the rate of placebo) reported in pediatric patients with bipolar I disorder treated with asenapine sublingual tablets were somnolence, dizziness, dysgeusia, oral hypoesthesia, nausea, increased appetite, fatigue, and increased weight. No new major safety findings were reported from a 50-week, open-label, uncontrolled safety trial.
A total of 651 pediatric patients were treated with asenapine sublingual tablets. Of these patients, 352 pediatric patients were treated with asenapine sublingual tablets for at least 180 days and 58 pediatric patients treated with asenapine sublingual tablets had at least 1 year of exposure. The safety of asenapine sublingual tablets was evaluated in 403 pediatric patients with bipolar I disorder who participated in a 3-week, placebo-controlled, double-blind trial, of whom 302 patients received asenapine sublingual tablets at fixed doses ranging from 2.5 mg to 10 mg twice daily.
The stated frequencies of adverse reactions represent the proportion of individuals who experienced a treatment- emergent adverse event of the type listed. A reaction was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Patients with Schizophrenia: The following findings are based on the short-term placebo-controlled pre-marketing trials for schizophrenia (a pool of three 6-week fixed-dose trials and one 6-week flexible-dose trial) in which sublingual asenapine sublingual tablets was administered in doses ranging from 5 to 10 mg twice daily.
Adverse Reactions Occurring at an Incidence of 2% or More in asenapine sublingual tablets -Treated Patients with Schizophrenia: Adverse reactions associated with the use of asenapine sublingual tablets (incidence of 2% or greater, rounded to the nearest percent, and asenapine sublingual tablets incidence greater than placebo) that occurred during acute therapy (up to 6-weeks in patients with schizophrenia) are shown in Table 8.
| System Organ Class/ Preferred Term | Placebo N=378 % | Asenapine Sublingual Tablets 5 mg twice daily N=274 % | Asenapine Sublingual Tablets 10mg twice daily N=208 % | All Asenapine Sublingual Tablets*
5mg or 10 mg twice daily N=572 % |
|---|---|---|---|---|
|
||||
| Gastrointestinal disorders | ||||
| Constipation | 6 | 7 | 4 | 5 |
| Dry mouth | 1 | 3 | 1 | 2 |
| Oral hypoesthesia | 1 | 6 | 7 | 5 |
| Salivary hypersecretion | 0 | <1 | 4 | 2 |
| Stomach discomfort | 1 | <1 | 3 | 2 |
| Vomiting | 5 | 4 | 7 | 5 |
| General disorders | ||||
| Fatigue | 3 | 4 | 3 | 3 |
| Irritability | <1 | 2 | 1 | 2 |
| Investigations | ||||
| Increased weight | <1 | 2 | 2 | 3 |
| Metabolism disorders | ||||
| Increased appetite | <1 | 3 | 0 | 2 |
| Nervous system disorders | ||||
| Akathisia† | 3 | 4 | 11 | 6 |
| Dizziness | 4 | 7 | 3 | 5 |
| Extrapyramidal symptoms (excluding akathisia)‡ | 7 | 9 | 12 | 10 |
| Somnolence§ | 7 | 15 | 13 | 13 |
| Psychiatric disorders | ||||
| Insomnia | 13 | 16 | 15 | 15 |
| Vascular disorders | ||||
| Hypertension | 2 | 2 | 3 | 2 |
Monotherapy in Adult Patients with Bipolar Mania: The following findings are based on the short-term placebo-controlled trials for bipolar mania (a pool of two 3-week flexible-dose trials and one 3-week fixed-dose trial) in which sublingual asenapine sublingual tablets was administered in doses of 5 mg or 10 mg twice daily.
Adverse Reactions Occurring at an Incidence of 2% or More Among asenapine sublingual tablets -Treated (Monotherapy) patients with Bipolar I Disorder: Adverse reactions associated with the use of asenapine sublingual tablets (incidence of 2% or greater, rounded to the nearest percent, and asenapine sublingual tablets incidence greater than placebo) that occurred during acute monotherapy (up to 3-weeks in patients with bipolar mania) are shown in Table 9.
| (Fixed Dose Study) | All Placebo* | All Asenapine Sublingual Tablets 5 mg or 10 mg twice daily† | |||
|---|---|---|---|---|---|
| System Organ Class/Preferred Term | Placebo | Asenapine Sublingual Tablets 5 mg twice daily | Asenapine Sublingual Tablets 10 mg twice daily | ||
| N=126 % | N=122 % | N=119 % | N=329 % | N=620 % |
|
|
|||||
| Gastrointestinal disorders | |||||
| Oral Hypoesthesia‡ | 2 | 13 | 24 | 1 | 10 |
| Nausea | 3 | 4 | 5 | 5 | 5 |
| Constipation | 2 | 4 | 3 | 4 | 4 |
| Dyspepsia§ | 6 | 4 | 5 | 4 | 4 |
| Vomiting | 2 | 1 | 3 | 3 | 3 |
| Abdominal Pain¶ | 0 | 2 | 3 | 3 | 3 |
| Dry Mouth | 5 | 3 | 1 | 2 | 3 |
| Toothache | 1 | 2 | 2 | 2 | 3 |
| General disorders | |||||
| Fatigue# | 2 | 2 | 5 | 2 | 4 |
| Infections and Infestations | |||||
| NasopharyngitisÞ | 2 | 1 | 5 | 2 | 3 |
| Investigations | |||||
| Weight Increase | 1 | 0 | 1 | 1 | 3 |
| Alanine Aminotransferase Increase | 0 | 0 | 3 | 0 | 1 |
| Metabolism disorders | |||||
| Increased appetite | 2 | 1 | 6 | 2 | 4 |
| Musculoskeletal and connective tissue disorders | |||||
| Arthralgia | 1 | 1 | 2 | 1 | 2 |
| Nervous system disorders | |||||
| Somnolenceß | 4 | 20 | 26 | 5 | 23 |
| Dizziness | 5 | 3 | 5 | 4 | 8 |
| Extrapyramidal symptoms (excluding akathisia)à | 7 | 7 | 11 | 4 | 8 |
| Akathisia | 1 | 4 | 15 | 2 | 6 |
| Dysgeusia | 0 | 3 | 9 | <1 | 4 |
| Psychiatric Disorders | |||||
| Bipolar Disorder/Maniaè | 3 | 8 | 3 | 5 | 6 |
| Agitation | 1 | 4 | 3 | 3 | 4 |
| Anxiety | 3 | 0 | 3 | 2 | 3 |
Monotherapy in Pediatric Patients with Bipolar Mania: The following findings are based on a 3-week, placebo-controlled trial for bipolar mania in which asenapine sublingual tablets was administered at doses of 2.5 mg, 5 mg, or 10 mg twice daily.
Adverse Reactions Occurring with Asenapine Sublingual Tablets at an Incidence of 2% or More in Asenapine Sublingual Tablets-treated Bipolar I Patients: Adverse reactions associated with the use of asenapine sublingual tablets (incidence of ≥2% in any asenapine sublingual tablets dose group and greater than placebo) that occurred during acute therapy are shown in Table 10.
| System Organ Class/ AE Preferred Term | Placebo | Asenapine sublingual tablets 2.5 mg twice daily | Asenapine sublingual tablets 5 mg twice daily | Asenapine sublingual tablets 10 mg twice daily | All Asenapine sublingual tablets 2.5, 5, and 10 mg |
|---|---|---|---|---|---|
| N=101% | N=104% | N=99% | N=99% | N=302% | |
|
|||||
| Cardiac Disorders | |||||
| Tachycardia* | 0 | 3 | 0 | 1 | 1 |
| Gastrointestinal Disorders | |||||
| Oral hypoesthesia† | 4 | 25 | 25 | 30 | 27 |
| Nausea | 3 | 6 | 6 | 6 | 6 |
| Vomiting | 3 | 4 | 4 | 4 | 4 |
| Abdominal pain‡ | 7 | 9 | 3 | 5 | 6 |
| Glossodynia | 0 | 0 | 2 | 0 | 1 |
| General Disorders and Administrative Site Disorders | |||||
| Fatigue§ | 5 | 4 | 8 | 14 | 9 |
| Irritability | 1 | 1 | 1 | 2 | 1 |
| Injury, Poisoning, and Procedural Complications | |||||
| Muscle strain | 0 | 0 | 0 | 2 | 1 |
| Investigations | |||||
| Increased weight | 0 | 6 | 2 | 2 | 3 |
| Hyperinsulinemia¶ | 0 | 1 | 3 | 1 | 2 |
| ALT increased | 0 | 0 | 0 | 2 | 1 |
| AST increased | 0 | 0 | 0 | 2 | 1 |
| Metabolism and Nutrition Disorders | |||||
| Increased appetite | 2 | 10 | 9 | 6 | 8 |
| Dehydration | 1 | 0 | 2 | 0 | 1 |
| Musculoskeletal and Connective Tissue Disorders | |||||
| Myalgia | 0 | 0 | 2 | 1 | 1 |
| Nervous System Disorders | |||||
| Somnolence# | 12 | 46 | 53 | 49 | 49 |
| Headache | 6 | 8 | 11 | 9 | 9 |
| Dizziness | 3 | 6 | 10 | 5 | 7 |
| Dysgeusia | 2 | 4 | 5 | 9 | 6 |
| Akathisia | 0 | 2 | 2 | 1 | 2 |
| Parkinsonism | 0 | 1 | 0 | 2 | 1 |
| Psychiatric Disorders | |||||
| Insomnia | 3 | 3 | 4 | 3 | 3 |
| Suicidal ideation | 1 | 4 | 1 | 3 | 3 |
| Anger | 0 | 0 | 0 | 2 | 1 |
| Reproductive System and Breast Disorders | |||||
| Dysmenorrhea | 1 | 0 | 2 | 0 | 1 |
| Respiratory, Thoracic, and Mediastinal Disorders | |||||
| Oropharyngeal pain | 2 | 0 | 3 | 1 | 1 |
| Nasal congestion | 1 | 0 | 2 | 0 | 1 |
| Dyspnea | 0 | 0 | 2 | 0 | 1 |
| Skin and Subcutaneous Tissue Disorders | |||||
| Rash | 1 | 0 | 1 | 2 | 1 |
Adjunctive Therapy in Adult Patients with Bipolar Mania: The following findings are based on a 12 week placebo-controlled trial (with a 3 week efficacy endpoint) in adult patients with bipolar mania in which asenapine sublingual tablets was administered in doses of 5 mg or 10 mg twice daily as adjunctive therapy with lithium or valproate.
Adverse Reactions Occurring at an Incidence of 2% or More Among Asenapine Sublingual Tablets -Treated (Adjunctive) Bipolar I Patients: Adverse reactions associated with the use of asenapine sublingual tablets (incidence of 2% or greater, rounded to the nearest percent, and asenapine sublingual tablets incidence greater than placebo) that occurred during acute adjunctive therapy at 3 weeks, a time when most of the patients were still participating in the trial, are shown in Table 11.
| System Organ Class/Preferred Term | Placebo N=166 % | Asenapine Sublingual Tablets 5 mg or 10 mg twice daily*
N=158 % |
|---|---|---|
|
||
| Gastrointestinal disorders | ||
| Dyspepsia | 2 | 3 |
| Oral hypoesthesia | 0 | 5 |
| General disorders | ||
| Fatigue | 2 | 4 |
| Edema peripheral | <1 | 3 |
| Investigations | ||
| Increased weight | 0 | 3 |
| Nervous system disorders | ||
| Dizziness | 2 | 4 |
| Other extrapyramidal symptoms (excluding akathisia)† | 5 | 6 |
| Somnolence‡ | 10 | 22 |
| Psychiatric disorders | ||
| Insomnia | 8 | 10 |
| Vascular disorders | ||
| Hypertension | <1 | 3 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of asenapine sublingual tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to establish a causal relationship to drug exposure. In many cases, the occurrence of these adverse reactions led to discontinuation of therapy.
- Application site reactions, primarily in the sublingual area, have been reported. These application site reactions included oral ulcers, blisters, peeling/sloughing, and inflammation.
- Choking has been reported by patients, some of whom may have also experienced oropharyngeal muscular dysfunction or hypoesthesia.
7. Drug Interactions
7.1 Drugs Having Clinically Important Drug Interactions with Asenapine Sublingual Tablets
| Concomitant Drug Name or Drug Class | Clinical Rationale | Clinical Recommendation |
|---|---|---|
| Antihypertensive Drugs | Because of its α1-adrenergic antagonism with potential for inducing hypotension, asenapine sublingual tablets may enhance the effects of certain antihypertensive agents [see Warnings and Precautions (5.7)]. | Monitor blood pressure and adjust dosage of antihypertensive drug accordingly. |
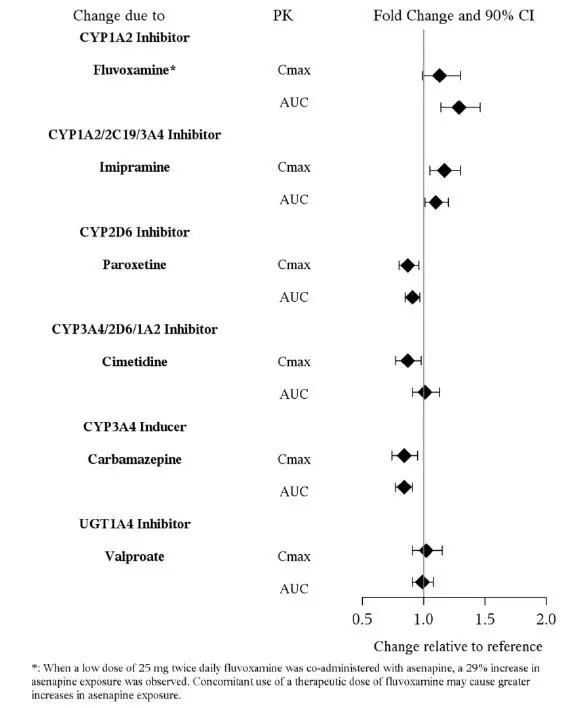
| Strong CYP1A2 Inhibitors (e.g., Fluvoxamine) | Asenapine sublingual tablets are metabolized by CYP1A2. Marginal increase of asenapine exposure was observed when asenapine sublingual tablets are used with fluvoxamine at 25 mg administered twice daily [see Clinical Pharmacology (12.3)]. However, the tested fluvoxamine dose was suboptimal. Full therapeutic dose of fluvoxamine is expected to cause a greater increase in asenapine exposure. | Dosage reduction for asenapine sublingual tablets based on clinical response may be necessary. |
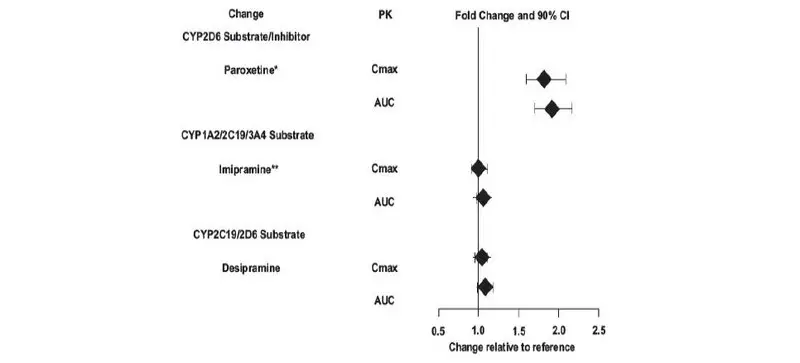
| CYP2D6 substrates and inhibitors (e.g., paroxetine) | Asenapine sublingual tablets may enhance the inhibitory effects of paroxetine on its own metabolism. Concomitant use of paroxetine with asenapine sublingual tablets increased the paroxetine exposure by 2-fold as compared to use paroxetine alone [see Clinical Pharmacology (12.3)]. | Reduce paroxetine dose by half when paroxetine is used in combination with asenapine sublingual tablets. |
7.2 Drugs Having No Clinically Important Interactions with Asenapine Sublingual Tablets
No dosage adjustment of asenapine sublingual tablets are necessary when administered concomitantly with paroxetine (see Table 12 in Drug Interactions (7.1) for paroxetine dosage adjustment), imipramine, cimetidine, valproate, lithium, or a CYP3A4 inducer (e.g., carbamazepine, phenytoin, rifampin).
In addition, valproic acid and lithium pre-dose serum concentrations collected from an adjunctive therapy study were comparable between asenapine-treated patients and placebo-treated patients indicating a lack of effect of asenapine on valproic and lithium plasma levels.
8. Use In Specific Populations
8.4 Pediatric Use
Safety and efficacy of asenapine sublingual tablets in pediatric patients below the age of 10 years of age have not been evaluated.
8.5 Geriatric Use
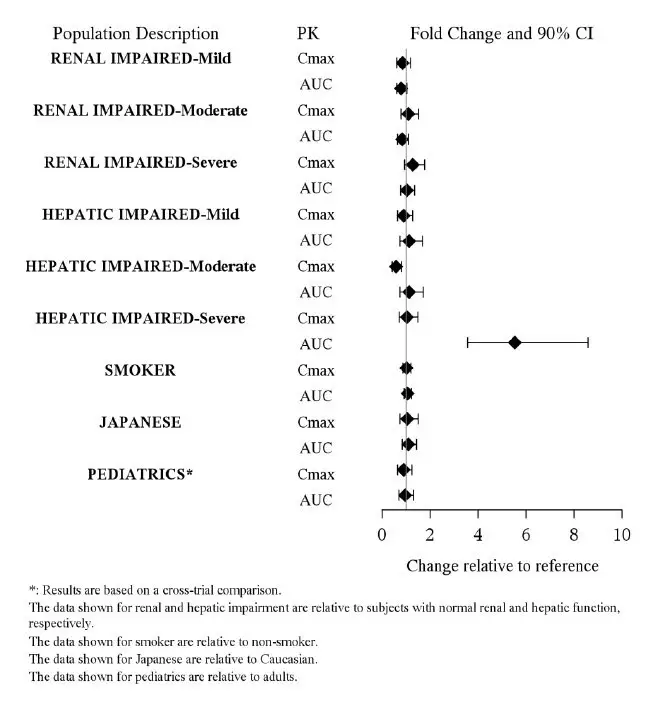
Clinical studies of asenapine sublingual tablets in the treatment of schizophrenia and bipolar mania did not include sufficient numbers of patients aged 65 and over to determine whether or not they respond differently than younger patients. Of the approximately 2,250 patients in pre-marketing clinical studies of asenapine sublingual tablets, 1.1% (25) were 65 years of age or over. Multiple factors that might increase the pharmacodynamic response to asenapine sublingual tablets, causing poorer tolerance or orthostasis, could be present in elderly patients, and these patients should be monitored carefully. Based on a pharmacokinetic study in elderly patients, dosage adjustments are not recommended based on age alone [see Clinical Pharmacology (12.3)].
Elderly patients with dementia-related psychosis treated with asenapine sublingual tablets are at an increased risk of death compared to placebo. Asenapine sublingual tablets are not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
8.6 Renal Impairment
No dosage adjustment for asenapine sublingual tablets is required on the basis of a patient's renal function (mild to severe renal impairment, glomerular filtration rate between 15 and 90 mL/minute). The exposure of asenapine was similar among subjects with varying degrees of renal impairment and subjects with normal renal function [see Clinical Pharmacology (12.3)]. The effect of renal function on the excretion of other metabolites and the effect of dialysis on the pharmacokinetics of asenapine has not been studied.
8.7 Hepatic Impairment
Asenapine sublingual tablets are contraindicated in patients with severe hepatic impairment (Child-Pugh C) because asenapine exposure is 7-fold higher in subjects with severe hepatic impairment than the exposure observed in subjects with normal hepatic function.
No dosage adjustment for asenapine sublingual tablets are required in patients with mild to moderate hepatic impairment (Child-Pugh A and B) because asenapine exposure is similar to that in subjects with normal hepatic function [see Contraindications (4) and Clinical Pharmacology (12.3)].
9. Drug Abuse and Dependence
9.2 Abuse
Asenapine sublingual tablets have not been systematically studied in animals or humans for their abuse potential or their ability to induce tolerance or physical dependence. Thus, it is not possible to predict the extent to which a CNS-active drug will be misused, diverted and/or abused once it is marketed. Patients should be evaluated carefully for a history of drug abuse, and such patients should be observed carefully for signs that they are misusing or abusing asenapine sublingual tablets (e.g., drug-seeking behavior, increases in dose).
11. Asenapine Tablets Description
Asenapine sublingual tablets contain asenapine maleate which is an atypical antipsychotic that is available for sublingual administration. Asenapine belongs to the class dibenzo-oxepino pyrroles. The chemical designation is (3aRS,12bRS)-5-Chloro-2-methyl-2,3,3a,12b-tetrahydro-1Hdibenzo[2,3:6,7]oxepino[4,5-c]pyrrole (2Z)-2-butenedioate (1:1). Its molecular formula is C17H16ClNO∙C4H4O4 and its molecular weight is 401.84 (free base: 285.8). The chemical structure is:
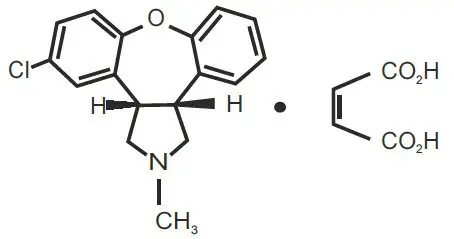
Asenapine maleate is a white to off-white powder.
Asenapine sublingual tablets (Unflavored) are supplied for sublingual administration in tablets containing 5 mg or 10 mg asenapine; inactive ingredients include aspartame, colloidal silicon dioxide, crospovidone, low substituted hydroxypropyl cellulose, microcrystalline cellulose, magnesium stearate and povidone.
Asenapine sublingual tablets, black cherry flavor, are supplied for sublingual administration in tablets containing 2.5 mg, 5 mg or 10 mg asenapine; inactive ingredients include aspartame, colloidal silicon dioxide, crospovidone, low substituted hydroxypropyl cellulose, microcrystalline cellulose, magnesium stearate and povidone and black cherry flavor (components are nature identical flavorings, natural flavoring substances, natural flavoring complexes, maize maltodextrin and moisture).
12. Asenapine Tablets - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of asenapine in schizophrenia and bipolar I disorder, is unknown. It has been suggested that the efficacy of asenapine in schizophrenia could be mediated through a combination of antagonist activity at D2 and 5-HT2A receptors.
12.2 Pharmacodynamics
Asenapine exhibits high affinity for serotonin 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5A, 5-HT6, and 5-HT7 receptors (Ki values of 2.5, 2.7, 0.07, 0.18, 0.03, 1.6, 0.25, and 0.11 nM, respectively), dopamine D2A, D2B, D3, D4, and D1 receptors (Ki values of 1.3, 1.4, 0.42, 1.1, and 1.4 nM, respectively), α1A, α2A, α2B, and α2C-adrenergic receptors (Ki values of 1.2, 1.2, 0.33 and 1.2 nM, respectively), and histamine H1 receptors (Ki value 1.0 nM), and moderate affinity for H2 receptors (Ki value of 6.2 nM). In in vitro assays asenapine acts as an antagonist at these receptors. Asenapine has no appreciable affinity for muscarinic cholinergic receptors (e.g., Ki value of 8,128 nM for M1).
12.3 Pharmacokinetics
Following a single 5 mg dose of asenapine sublingual tablets, the mean Cmax was approximately 4 ng/mL and was observed at a mean tmax of 1 hour. Elimination of asenapine is primarily through direct glucuronidation by UGT1A4 and oxidative metabolism by cytochrome P450 isoenzymes (predominantly CYP1A2). Following an initial more rapid distribution phase, the mean terminal half-life is approximately 24 hrs. With multiple-dose twice-daily dosing, steady-state is attained within 3 days. Overall, steady-state asenapine pharmacokinetics are similar to single-dose pharmacokinetics.
14. Clinical Studies
Efficacy of asenapine sublingual tablets was established in the following trials:
- Two fixed-dose, short-term trials and one flexible-dose, maintenance trial in adult patients with schizophrenia as monotherapy [see Clinical Studies (14.1)]
- One fixed-dose and two flexible-dose, short-term trials of monotherapy in adults with manic or mixed episodes associated with bipolar I disorder [see Clinical Studies (14.2)]
- One flexible-dose, maintenance trial of monotherapy in adults with bipolar I disorder [see Clinical Studies (14.2)]
- One fixed-dose, short term trial of monotherapy in children (10 to 17 years) with manic or mixed episodes associated with bipolar I disorder [see Clinical Studies (14.2)]
- One flexible-dose, short-term trial in adult patients with manic or mixed episode associated with bipolar I disorder as adjunctive treatment to lithium or valproate [see Clinical Studies (14.2)]
14.1 Schizophrenia
The efficacy of asenapine sublingual tablets in the treatment of schizophrenia in adults was evaluated in three fixed-dose, short-term (6 week), randomized, double-blind, placebo-controlled, and active-controlled (haloperidol, risperidone, and olanzapine) trials of adult patients who met DSM-IV criteria for schizophrenia and were having an acute exacerbation of their schizophrenic illness. In two of the three trials asenapine sublingual tablets demonstrated superior efficacy to placebo. In a third trial, asenapine sublingual tablets could not be distinguished from placebo; however, an active control in that trial was superior to placebo.
In the two positive trials for asenapine sublingual tablets, the primary efficacy rating scale was the Positive and Negative Syndrome Scale (PANSS). The PANSS is a 30 item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210. The primary endpoint was change from baseline to endpoint on the PANSS total score. The results of the asenapine sublingual tablets trials in schizophrenia follow:
In trial 1, a 6-week trial (n=174), comparing asenapine sublingual tablets (5 mg twice daily) to placebo, asenapine sublingual tablets 5 mg twice daily was statistically superior to placebo on the PANSS total score (Trial 1 in Table 13).
In trial 2, a 6-week trial (n=448), comparing two fixed doses of asenapine sublingual tablets (5 mg and 10 mg twice daily) to placebo, asenapine sublingual tablets 5 mg twice daily was statistically superior to placebo on the PANSS total score. Asenapine sublingual tablets 10 mg twice daily showed no added benefit compared to 5 mg twice daily and was not significantly different from placebo (Trial 2 in Table 13).
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex or race.
| Trial Number | Treatment Group | Primary Efficacy Measure: PANSS Total Score | ||
|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference* (95% CI) | ||
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiple comparisons. | ||||
|
||||
| Trial 1 | Asenapine Sublingual Tablets 5 mg† twice daily | 96.5 (16.4) | -14.4 (2.6) | -9.7 (-17.6, -1.8) |
| Placebo | 92.4 (14.9) | -4.6 (2.5) | - | |
| Trial 2 | Asenapine Sublingual Tablets 5 mg† twice daily | 89.2 (12.0) | -16.2 (1.7) | -5.5 (-10.7, -0.2) |
| Asenapine Sublingual Tablets 10 mg twice daily | 89.1 (12.9) | -14.9 (1.7) | -4.1 (-9.4, 1.2) | |
| Placebo | 88.9 (11.7) | -10.7 (1.6) | - | |
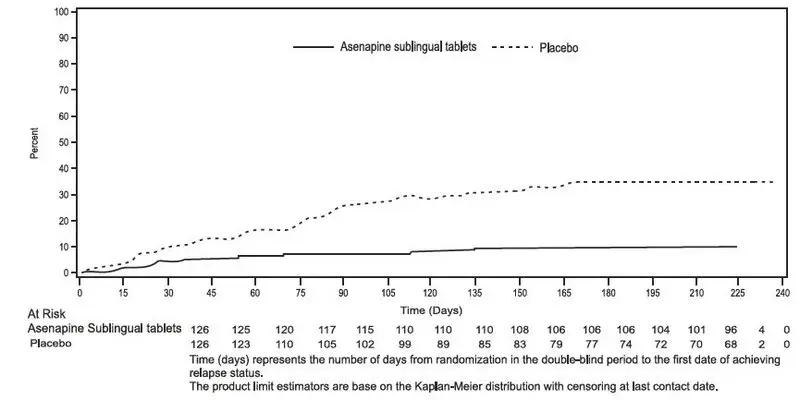
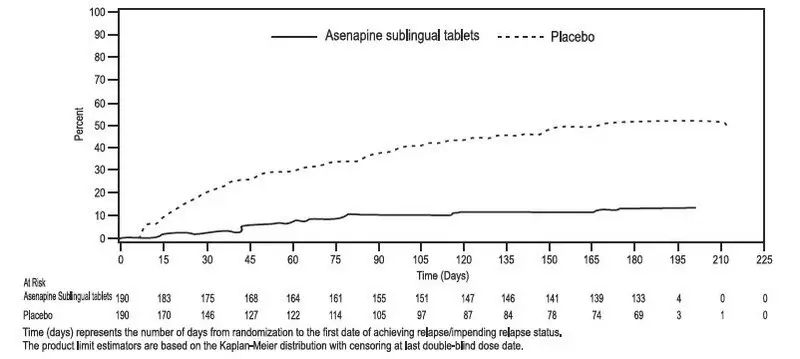
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. All patients were initially administered 5 mg twice daily for 1 week and then titrated up to 10 mg twice daily. A total of 700 patients entered open-label treatment with asenapine sublingual tablets for a period of 26 weeks. Of these, a total of 386 patients who met pre-specified criteria for continued stability (mean length of stabilization was 22 weeks) were randomized to a double-blind, placebo-controlled, randomized withdrawal phase. Asenapine sublingual tablets was statistically superior to placebo in time to relapse or impending relapse defined as increase in PANSS ≥20% from baseline and a Clinical Global Impression–Severity of Illness (CGI-S) score ≥4 (at least 2 days within 1 week) or PANSS score ≥5 on "hostility" or "uncooperativeness" items and CGI-S score ≥4 (≥2 days within a week), or PANSS score ≥5 on any two of the following items: "unusual thought content," "conceptual disorganization," or "hallucinatory behavior" items, and CGI-S score ≥4 (≥2 days within 1 week) or investigator judgment of worsening symptoms or increased risk of violence to self (including suicide) or other persons. The Kaplan-Meier curves of the time to relapse or impending relapse during the double-blind, placebo-controlled, randomized withdrawal phase of this trial for asenapine sublingual tablets and placebo are shown in Figure 4.
Figure 4: Kaplan-Meier Estimation of Percent Relapse/Impending Relapse for Asenapine Sublingual Tablets and placebo
14.2 Bipolar I Disorder
Adjunctive Therapy: The efficacy of asenapine sublingual tablets as an adjunctive therapy in acute mania was established in a 12-week, placebo-controlled trial with a 3-week primary efficacy endpoint involving 326 adult patients with a manic or mixed episode of Bipolar I Disorder, with or without psychotic features, who were partially responsive to lithium or valproate monotherapy after at least 2 weeks of treatment. All patients randomized to asenapine sublingual tablets were initially administered 5 mg twice daily, and the dose could be adjusted within the dose range of 5 to 10 mg twice daily from Day 2 onward based on efficacy and tolerability. Asenapine was statistically superior to placebo in the reduction of manic symptoms (measured by the YMRS total score) as an adjunctive therapy to lithium or valproate monotherapy at Week 3 (Trial 5 Adjunctive in Table 14).
| Study Number | Treatment Group | Primary Efficacy Measure: YMRS Total Score | ||
|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Difference* (95% CI) | ||
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiple comparisons. | ||||
|
||||
| Trial 1 | Asenapine sublingual tablets 5 - 10 mg† twice daily | 29.4 (6.7) | -11.5 (0.8) | -3.7 (-6.6, -0.7) |
| Placebo | 28.3 (6.3) | -7.8 (1.1) | - | |
| Trial 2 | Asenapine sublingual tablets 5 - 10 mg† twice daily | 28.3 (5.5) | -10.8 (0.8) | -5.3 (-8.0, -2.5) |
| Placebo | 29.0 (6.1) | -5.5 (1.0) | - | |
| Trial 3 | Asenapine sublingual tablets 5 mg† twice daily | 29.7 (5.9) | -14.4 (1.0) | -3.5 (-6.3, -0.7) |
| Asenapine sublingual tablets 10 mg† twice daily | 30.2 (5.4) | -14.9 (1.0) | -4.0 (-6.9, -1.2) | |
| Placebo | 30.0 (5.6) | -10.9 (1.0) | - | |
| Trial 4 (Pediatric 10 to 17 years) | Asenapine sublingual tablets 2.5 mg† twice daily | 29.5 (5.7) | -12.8 (0.8) | -3.2 (-5.6, -0.8) |
| Asenapine sublingual tablets 5 mg† twice daily | 30.4 (5.9) | -14.9 (0.8) | -5.3 (-7.7, -2.9) | |
| Asenapine sublingual tablets 10 mg† twice daily | 30.1 (5.7) | -15.8 (0.9) | -6.2 (-8.6, -3.8) | |
| Placebo | 30.1 (5.7) | - 9.6 (0.9) | - | |
| Trial 5 (Adjunctive) | Asenapine sublingual tablets 5 - 10 mg† twice daily + lithium/ Valproate | 28.0 (5.6) | -10.3 (0.8) | -2.4 (-4.4, -0.3) |
| Lithium/Valproate | 28.2 (5.8) | -7.9 (0.8) | - | |
16. How is Asenapine Tablets supplied
Asenapine sublingual tablets are supplied as:
| 5 mg Tablets | |
| Round, white, uncoated tablets debossed "B" on one side and "1" on the other side. | |
| Child-resistant packaging | |
| Box of 60 - 6 blisters with 10 tablets | NDC 51991-359-60 |
| Hospital Unit Dose | |
| Box of 100 - 10 blisters with 10 tablets | NDC 51991-359-11 |
| 10 mg Tablets | |
| Round, white, uncoated tablets debossed "B" on one side and "2" on the other side. | |
| Child-resistant packaging | |
| Box of 60 - 6 blisters with 10 tablets | NDC 51991-361-60 |
| Hospital Unit Dose | |
| Box of 100 - 10 blisters with 10 tablets | NDC 51991-361-11 |
| 2.5 mg Tablets, black cherry flavor | |
| White colored, round shaped, uncoated tablets debossed with "B" on one side and "5" on the other side. | |
| Child-resistant packaging | |
| Box of 60 - 6 blisters with 10 tablets | NDC 51991-928-60 |
| Hospital Unit Dose | |
| Box of 100 - 10 blisters with 10 tablets | NDC 51991-928-11 |
| 5 mg Tablets, black cherry flavor | |
| Round, white, uncoated tablets debossed "B" on one side and "3" on the other side. | |
| Child-resistant packaging | |
| Box of 60 - 6 blisters with 10 tablets | NDC 51991-358-60 |
| Hospital Unit Dose | |
| Box of 100 - 10 blisters with 10 tablets | NDC 51991-358-11 |
| 10 mg Tablets, black cherry flavor | |
| Round, white, uncoated tablets debossed "B" on one side and "4" on the other side. | |
| Child-resistant packaging | |
| Box of 60 - 6 blisters with 10 tablets | NDC 51991-360-60 |
| Hospital Unit Dose | |
| Box of 100 - 10 blisters with 10 tablets | NDC 51991-360-11 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
| ASENAPINE
asenapine maleate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ASENAPINE
asenapine maleate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ASENAPINE
asenapine maleate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ASENAPINE
asenapine maleate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ASENAPINE
asenapine maleate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CENTAUR PHARMACEUTICALS PVT. LTD., (Pune) | 675596622 | MANUFACTURE(51991-358, 51991-359, 51991-360, 51991-361, 51991-928) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MSN Laboratories Private Limited | 650786952 | MANUFACTURE(51991-358, 51991-359, 51991-360, 51991-361, 51991-928) | |