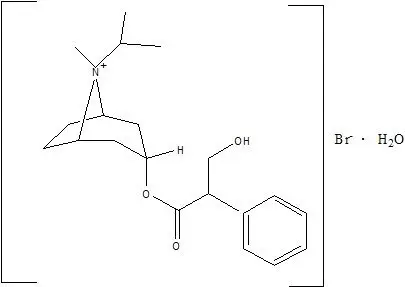
Drug Detail:Atrovent nasal (Ipratropium nasal [ ip-ra-tro-pee-um ])
Drug Class: Nasal antihistamines and decongestants
Related/similar drugs
ipratropium nasal, doxylamine, triprolidine, Vicks NyQuil Severe Cold & Flu, Coricidin HBP Cold & Flu, Capron DMTClinical Studies
Precautions
General
- 1.
- Effects Seen with Anticholinergic Drugs: ATROVENT Nasal Spray 0.03% should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia, or bladder neck obstruction, particularly if they are receiving an anticholinergic by another route.
- 2.
- Use in Hepatic or Renal Disease: ATROVENT Nasal Spray 0.03% has not been studied in patients with hepatic or renal insufficiency. It should be used with caution in those patient populations.
Adverse Reactions/Side Effects
| Atrovent®
(ipratropium bromide) | Vehicle Control | |||
|---|---|---|---|---|
| Nasal Spray 0.03% | ||||
| (n=356) | (n=347) | |||
| Incidence % | Discontinued % | Incidence % | Discontinued % | |
| Headache | 9.8 | 0.6 | 9.2 | 0.0 |
| Upper respiratory | ||||
| tract infection | 9.8 | 1.4 | 7.2 | 1.4 |
| Epistaxis1 | 9.0 | 0.3 | 4.6 | 0.3 |
| Rhinitis* | ||||
| Nasal dryness | 5.1 | 0.0 | 0.9 | 0.3 |
| Nasal irritation2 | 2.0 | 0.0 | 1.7 | 0.6 |
| Other nasal symptoms3 | 3.1 | 1.1 | 1.7 | 0.3 |
| Pharyngitis | 8.1 | 0.3 | 4.6 | 0.0 |
| Nausea | 2.2 | 0.3 | 0.9 | 0.0 |
There were infrequent reports of skin rash in both the controlled and uncontrolled clinical studies.
How is Atrovent Nasal Spray supplied
Patient's Instructions for Use
Atrovent®
(ipratropium bromide)
Nasal Spray 0.03%
Read complete instructions carefully before using.
In order to ensure proper dosing, do not attempt to change the size of the spray opening.
Read complete instructions carefully and use only as directed.
- Remove the clear plastic dust cap and the green safety clip
from the nasal spray pump (Figure 1). The safety
clip prevents the accidental discharge of the spray in your pocket
or purse.
Figure 1
- The nasal spray pump must be primed before Atrovent® (ipratropium bromide) Nasal Spray 0.03% is used for
the first time. To prime the pump, hold the bottle with your thumb
at the base and your index and middle fingers on the white shoulder
area. Make sure the bottle points upright and away from your eyes.
Press your thumb firmly and quickly against the bottle seven times
(Figure 2). The pump is now primed and can
be used. Your pump should not have to be reprimed unless you have
not used the medication for more than 24 hours; repriming the pump
will only require two sprays. If you have not used your nasal spray
for more than seven days, repriming the pump will require seven sprays.
Figure 2
- Before using ATROVENT Nasal Spray 0.03%, blow your nose gently to clear your nostrils if necessary.
- Close one nostril by gently placing your finger against
the side of your nose, tilt your head slightly forward and, keeping
the bottle upright, insert the nasal tip into the other nostril (Figure 3). Point the tip toward the back and outer side of the nose.
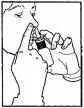
Figure 3
- Press firmly and quickly upwards with the thumb at the base while holding the white shoulder portion of the pump between your index and middle fingers. Following each spray, sniff deeply and breathe out through your mouth.
- After spraying the nostril and removing the unit, tilt your head backwards for a few seconds to let the spray spread over the back of the nose.
- Repeat steps 4 through 6 in the same nostril.
- Repeat steps 4 through 7 in the other nostril (i.e., two sprays per nostril).
- Replace the clear plastic dust cap and safety clip.
- At some time before the medication is completely used up, you should consult your physician or pharmacist to determine whether a refill is needed. You should not take extra doses or stop using Atrovent® (ipratropium bromide) Nasal Spray 0.03% without consulting your physician.
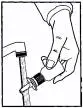
Figure 4
Should you experience excessive nasal dryness or episodes of nasal bleeding contact your doctor.
Distributed by:
Boehringer Ingelheim Pharmaceuticals,
Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer
Ingelheim International GmbH
Copyright 2011 Boehringer Ingelheim International
GmbH
ALL RIGHTS RESERVED
| ATROVENT
ipratropium bromide spray, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West-Ward Columbus Inc. | 058839929 | PACK(0597-0081) , LABEL(0597-0081) , ANALYSIS(0597-0081) , MANUFACTURE(0597-0081) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | ANALYSIS(0597-0081) , API MANUFACTURE(0597-0081) | |