Drug Detail:Avalide (Hydrochlorothiazide and irbesartan [ hye-droe-klor-oh-thye-a-zide-and-ir-be-sar-tan ])
Drug Class: Angiotensin II inhibitors with thiazides
Highlights of Prescribing Information
AVALIDE® (irbesartan and hydrochlorothiazide) tablets, for oral use
Initial U.S. Approval: 1997
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
- When pregnancy is detected, discontinue AVALIDE as soon as possible. (5.1, 8.1)
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1, 8.1)
Indications and Usage for Avalide
AVALIDE is a combination of irbesartan, an angiotensin II receptor antagonist, and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension:
- In patients not adequately controlled with monotherapy. (1)
- As initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals. (1)
Avalide Dosage and Administration
General Considerations
- Maximum effects within 2 to 4 weeks after dose change. (2.1)
- Renal impairment: Not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min). (2.1, 5.8)
Hypertension
- Initiate with 150/12.5 mg. Titrate to 300/12.5 mg then 300/25 mg if needed. (2.2)
- Replacement therapy: May be substituted for titrated components. (2.3)
Dosage Forms and Strengths
- 150 mg irbesartan/12.5 mg hydrochlorothiazide tablets (3)
- 300 mg irbesartan/12.5 mg hydrochlorothiazide tablets (3)
Contraindications
- Hypersensitivity to any component of this product. (4)
- Anuria. (4)
- Hypersensitivity to sulfonamide-derived drugs. (4)
- Do not coadminister aliskiren with AVALIDE in patients with diabetes. (4)
Warnings and Precautions
- Hypotension: Correct volume depletion prior to administration. (5.2)
- Impaired renal function. (5.7)
- Thiazide diuretics may cause an exacerbation or activation of systemic lupus erythematosus. (5.4)
- Acute angle-closure glaucoma, acute myopia, and choroidal effusion. (5.8)
Adverse Reactions/Side Effects
Most common adverse events (≥5% on AVALIDE and more often than on placebo) are dizziness, fatigue, and musculoskeletal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- NSAIDs and selective COX-2 inhibitors: Can reduce diuretic, natriuretic of diuretic, may lead to increased risk of renal impairment and reduced antihypertensive effect. Monitor renal function periodically. (7)
- Dual blockade of the renin-angiotensin system: Increased risk of renal impairment, hypotension, and hyperkalemia. (7)
- Antidiabetic drugs: Dosage adjustment of antidiabetic may be required. (7)
- Cholestyramine and colestipol: Reduced absorption of thiazides. (7)
- Lithium: Increases in serum lithium concentrations and lithium toxicity. (7)
- Carbamazepine: Increased risk of hyponatremia. (7)
Use In Specific Populations
- Lactation: Potential for adverse effects in infant. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
Full Prescribing Information
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue AVALIDE as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
1. Indications and Usage for Avalide
AVALIDE® (irbesartan and hydrochlorothiazide) tablets are indicated for the treatment of hypertension.
AVALIDE may be used in patients whose blood pressure is not adequately controlled on monotherapy.
AVALIDE may also be used as initial therapy in patients who are likely to need multiple drugs to achieve their blood pressure goals.
The choice of AVALIDE as initial therapy for hypertension should be based on an assessment of potential benefits and risks.
Patients with stage 2 (moderate or severe) hypertension are at relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and may be shaped by considerations such as the baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared with monotherapy.
Data from Studies V and VI [see Clinical Studies (14.2)] provide estimates of the probability of reaching a blood pressure goal with AVALIDE compared to irbesartan or hydrochlorothiazide (HCTZ) monotherapy. The relationship between baseline blood pressure and achievement of a SeSBP <140 or <130 mmHg or SeDBP <90 or <80 mmHg in patients treated with AVALIDE compared to patients treated with irbesartan or HCTZ monotherapy are shown in Figures 1a through 2b.
|
|
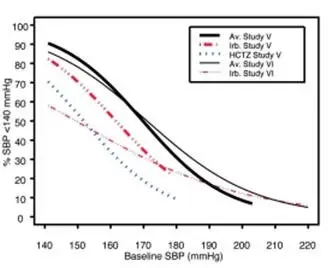 | 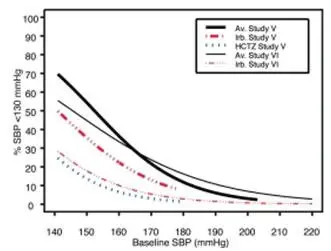 |
| Figure 1a: Probability of Achieving SBP <140 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* | Figure 1b: Probability of Achieving SBP <130 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* |
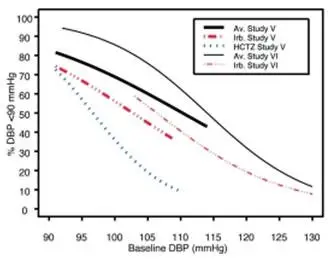 | 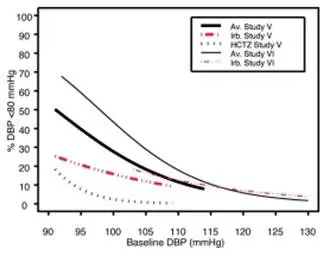 |
| Figure 2a: Probability of Achieving DBP <90 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* | Figure 2b: Probability of Achieving DBP <80 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7)* |
The above graphs provide a rough approximation of the likelihood of reaching a targeted blood pressure goal (e.g., Week 8 sitting systolic blood pressure ≤140 mmHg) for the treatment groups. The curve of each treatment group in each study was estimated by logistic regression modeling from all available data of that treatment group. The estimated likelihood at the right tail of each curve is less reliable due to small numbers of subjects with high baseline blood pressures.
For example, a patient with a blood pressure of 180/105 mmHg has about a 25% likelihood of achieving a goal of <140 mmHg (systolic) and 50% likelihood of achieving <90 mmHg (diastolic) on irbesartan alone (and lower still likelihoods on HCTZ alone).
The likelihood of achieving these goals on AVALIDE rises to about 40% (systolic) or 70% (diastolic).
2. Avalide Dosage and Administration
2.1 General Considerations
The side effects of irbesartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. [See Adverse Reactions (6).]
Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose.
AVALIDE may be administered with or without food.
AVALIDE may be administered with other antihypertensive agents.
2.2 Add-On Therapy
In patients not controlled on monotherapy with irbesartan or hydrochlorothiazide, the recommended doses of AVALIDE, in order of increasing mean effect, are (irbesartan and hydrochlorothiazide) 150/12.5 mg, 300/12.5 mg, and 300/25 mg. The largest incremental effect will likely be in the transition from monotherapy to 150/12.5 mg. [See Clinical Studies (14.2).]
2.4 Initial Therapy
The usual starting dose is AVALIDE 150/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of 300/25 mg once daily as needed to control blood pressure [see Clinical Studies (14.2)]. AVALIDE is not recommended as initial therapy in patients with intravascular volume depletion [see Warnings and Precautions (5.2)].
3. Dosage Forms and Strengths
AVALIDE® (irbesartan and hydrochlorothiazide) 150/12.5 mg and 300/12.5 mg film-coated tablets are peach, biconvex, and oval with a heart debossed on one side and "2875" or "2876" on the reverse side, respectively.
4. Contraindications
- AVALIDE is contraindicated in patients who are hypersensitive to any component of this product.
- Because of the hydrochlorothiazide component, this product is contraindicated in patients with anuria or hypersensitivity to other sulfonamide-derived drugs.
- Do not coadminister aliskiren with AVALIDE in patients with diabetes [see Drug Interactions (7)].
5. Warnings and Precautions
5.1 Fetal Toxicity
AVALIDE can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue AVALIDE as soon as possible [see Use in Specific Populations (8.1)].
Thiazides cross the placenta and use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
5.2 Hypotension in Volume or Salt-Depleted Patients
Excessive reduction of blood pressure was rarely seen in patients with uncomplicated hypertension treated with irbesartan alone (<0.1%) or with irbesartan and hydrochlorothiazide (approximately 1%). Initiation of antihypertensive therapy may cause symptomatic hypotension in patients with intravascular volume or sodium depletion, e.g., in patients treated vigorously with diuretics or in patients on dialysis. Such volume depletion should be corrected prior to administration of antihypertensive therapy.
If hypotension occurs, the patient should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.7 Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals [see Drug Interactions (7)]. In patients whose renal function may depend on the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with ACE inhibitors has been associated with oliguria and/or progressive azotemia and (rarely) with acute renal failure and/or death. Irbesartan would be expected to behave similarly. In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or BUN have been reported. There has been no known use of irbesartan in patients with unilateral or bilateral renal artery stenosis, but a similar effect should be anticipated.
Thiazides should be used with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of AVALIDE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to AVALIDE.
The following have been very rarely reported with irbesartan and hydrochlorothiazide monotherapies: urticaria, jaundice, hepatitis, thrombocytopenia, and impaired renal function including renal failure.
The following have been reported with irbesartan monotherapy: tinnitus, hyperkalemia, angioedema (involving swelling of the face, lips, pharynx, and/or tongue), anaphylactic reaction including anaphylactic shock, increased CPK, anemia, and hypoglycemia in diabetic patients.
The following have been reported with hydrochlorothiazide monotherapy: acute angle-closure glaucoma, acute myopia, and choroidal effusion.
6.3 Laboratory Abnormalities
In controlled clinical trials, clinically important changes in standard laboratory parameters were rarely associated with administration of AVALIDE.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen (BUN) or serum creatinine were observed in 2.3% and 1.1%, respectively, of patients with essential hypertension treated with AVALIDE alone. No patient discontinued taking AVALIDE due to increased BUN. One patient discontinued taking AVALIDE due to a minor increase in serum creatinine.
Liver Function Tests: Occasional elevations of liver enzymes and/or serum bilirubin have occurred. In patients with essential hypertension treated with AVALIDE alone, one patient was discontinued due to elevated liver enzymes.
Serum Electrolytes: [See Warnings and Precautions (5.2, 5.6).]
7. Drug Interactions
7.1 Nonsteroidal Anti-inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
7.2 Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin-receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function, and electrolytes in patients on AVALIDE and other agents that affect the RAS.
In most patients no benefit has been associated with using two RAS inhibitors concomitantly. In general, avoid combined use of RAS inhibitors.
Do not coadminister aliskiren with AVALIDE in patients with diabetes. Avoid use of aliskiren with AVALIDE in patients with renal impairment (GFR <60 mL/min).
7.3 Agents Increasing Serum Potassium
Coadministration of AVALIDE with other drugs that raise serum potassium levels may result in hyperkalemia, sometimes severe. Monitor serum potassium in such patients.
7.4 Antidiabetic Drugs (oral agents and insulin)
Dosage adjustment of the antidiabetic drug may be required when coadministered with hydrochlorothiazide.
7.5 Cholestyramine and Colestipol Resins
Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Stagger the dosage of hydrochlorothiazide and the resin such that AVALIDE is administered at least 4 hours before or 4 to 6 hours after the administration of the resin.
8. Use In Specific Populations
8.2 Lactation
There are no available data on the presence of irbesartan in human milk, effects on milk production, or the breastfed infant. Irbesartan or some metabolite of irbesartan is secreted in the milk of lactating rats. Thiazides appear in human milk [see Clinical Pharmacology (12.3)]. Because of the potential for adverse effects on the nursing infant, the use of AVALIDE in breastfeeding women is not recommended.
8.5 Geriatric Use
Of 1694 patients receiving AVALIDE in controlled clinical studies of hypertension, 264 (15.6%) were 65 years and over, while 45 (2.7%) were 75 years and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. [See Clinical Pharmacology (12.3) and Clinical Studies (14).]
11. Avalide Description
AVALIDE (irbesartan and hydrochlorothiazide) tablets are a combination of an angiotensin II receptor antagonist (AT1 subtype), irbesartan, and a thiazide diuretic, hydrochlorothiazide (HCTZ).
Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C25H28N6O, and its structural formula is:
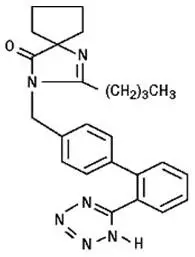
Irbesartan is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
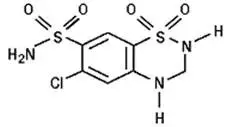
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water and freely soluble in sodium hydroxide solution.
AVALIDE is available for oral administration in film-coated tablets containing either 150 mg or 300 mg of irbesartan combined with 12.5 mg of hydrochlorothiazide. All dosage strengths contain the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hypromellose, magnesium stearate, silicon dioxide, ferric oxide red, ferric oxide yellow, polyethylene glycol, titanium dioxide, and carnauba wax.
12. Avalide - Clinical Pharmacology
14. Clinical Studies
14.1 Irbesartan Monotherapy
The antihypertensive effects of irbesartan were examined in 7 major placebo-controlled, 8 to 12-week trials in patients with baseline diastolic blood pressures of 95 to 110 mmHg. Doses of 1 to 900 mg were included in these trials in order to fully explore the dose-range of irbesartan. These studies allowed a comparison of once or twice-daily regimens at 150 mg/day, comparisons of peak and trough effects, and comparisons of response by gender, age, and race. Two of the 7 placebo-controlled trials identified above and 2 additional placebo-controlled studies examined the antihypertensive effects of irbesartan and hydrochlorothiazide in combination.
The 7 studies of irbesartan monotherapy included a total of 1915 patients randomized to irbesartan (1 to 900 mg) and 611 patients randomized to placebo. Once-daily doses of 150 to 300 mg provided statistically and clinically significant decreases in systolic and diastolic blood pressure with trough (24-hour post dose) effects after 6 to 12 weeks of treatment compared to placebo, of about 8 to 10/5 to 6 mmHg and 8 to 12/5 to 8 mmHg, respectively. No further increase in effect was seen at dosages greater than 300 mg. The dose-response relationships for effects on systolic and diastolic pressure are shown in Figures 3 and 4.
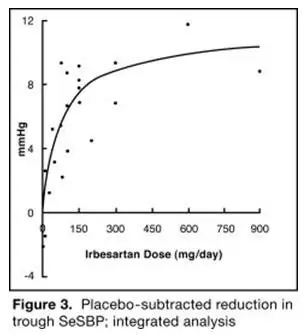 | 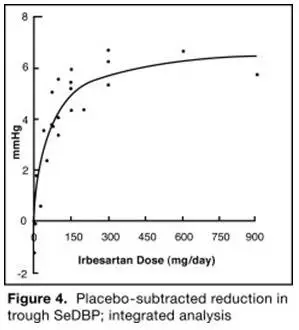 |
Once-daily administration of therapeutic doses of irbesartan gave peak effects at around 3 to 6 hours and, in one continuous ambulatory blood pressure monitoring study, again around 14 hours. This was seen with both once-daily and twice-daily dosing. Trough-to-peak ratios for systolic and diastolic response were generally between 60% and 70%. In a continuous ambulatory blood pressure monitoring study, once-daily dosing with 150 mg gave trough and mean 24-hour responses similar to those observed in patients receiving twice-daily dosing at the same total daily dose.
Analysis of age, gender, and race subgroups of patients showed that men and women, and patients over and under 65 years of age, had generally similar responses. Irbesartan was effective in reducing blood pressure regardless of race, although the effect was somewhat less in blacks (usually a low-renin population). Black patients typically show an improved response with the addition of a low dose diuretic (e.g., 12.5 mg hydrochlorothiazide).
The effect of irbesartan is apparent after the first dose and is close to the full observed effect at 2 weeks. At the end of the 8-week exposure, about 2/3 of the antihypertensive effect was still present 1 week after the last dose. Rebound hypertension was not observed. There was essentially no change in average heart rate in irbesartan-treated patients in controlled trials.
14.2 Irbesartan and Hydrochlorothiazide
The antihypertensive effects of AVALIDE (irbesartan and hydrochlorothiazide) tablets were examined in 4 placebo-controlled studies in patients with mild-moderate hypertension (mean seated diastolic blood pressure [SeDBP] between 90 and 110 mmHg), one study in patients with moderate hypertension (mean seated systolic blood pressure [SeSBP] 160 to 179 mmHg or SeDBP 100 to 109 mmHg), and one study in patients with severe hypertension (mean SeDBP ≥110 mmHg) of 8 to 12 weeks. These trials included 3149 patients randomized to fixed doses of irbesartan (37.5 to 300 mg) and concomitant hydrochlorothiazide (6.25 to 25 mg).
Study I was a factorial study that compared all combinations of irbesartan (37.5 mg, 100 mg, and 300 mg or placebo) and hydrochlorothiazide (6.25 mg, 12.5 mg, and 25 mg or placebo).
Study II compared the irbesartan and hydrochlorothiazide combinations of 75/12.5 mg and 150/12.5 mg to their individual components and placebo.
Study III investigated the ambulatory blood pressure responses to irbesartan and hydrochlorothiazide (75/12.5 mg and 150/12.5 mg) and placebo after 8 weeks of dosing.
Study IV investigated the effects of the addition of irbesartan (75 or 150 mg) in patients not controlled (SeDBP 93–120 mmHg) on hydrochlorothiazide (25 mg) alone. In Studies I–III, the addition of irbesartan 150 to 300 mg to hydrochlorothiazide doses of 6.25, 12.5, or 25 mg produced further dose-related reductions in blood pressure at trough of 8 to 10 mmHg/3 to 6 mmHg, similar to those achieved with the same monotherapy dose of irbesartan. The addition of hydrochlorothiazide to irbesartan produced further dose-related reductions in blood pressure at trough (24 hours post dose) of 5 to 6/2 to 3 mmHg (12.5 mg) and 7 to 11/4 to 5 mmHg (25 mg), also similar to effects achieved with hydrochlorothiazide alone. Once-daily dosing with 150 mg irbesartan and 12.5 mg hydrochlorothiazide, 300 mg irbesartan and 12.5 mg hydrochlorothiazide, or 300 mg irbesartan and 25 mg hydrochlorothiazide produced mean placebo-adjusted blood pressure reductions at trough (24 hours post dosing) of about 13 to 15/7 to 9 mmHg, 14/9 to 12 mmHg, and 19 to 21/11 to 12 mmHg, respectively. Peak effects occurred at 3 to 6 hours, with the trough-to-peak ratios >65%.
In Study IV, the addition of irbesartan (75–150 mg) gave an additive effect (systolic/diastolic) at trough (24 hours post dosing) of 11/7 mmHg.
16. How is Avalide supplied
16.1 How Supplied
AVALIDE® (irbesartan and hydrochlorothiazide) film-coated tablets have markings on both sides and are available in the strengths and packages listed in the following table:
| Tablet Strength (irbesartan and hydrochlorothiazide) | Film-Coated Tablet Color/Shape | Tablet Markings | Package Size | NDC Code |
|---|---|---|---|---|
| 150 mg and 12.5 mg | peach, biconvex, oval-shaped | heart debossed on one side and "2875" on the reverse | Bottles of 30 | 0024-5855-30 |
| 300 mg and 12.5 mg | peach, biconvex, oval-shaped | heart debossed on one side and "2876" on the reverse | Bottles of 30 | 0024-5856-30 |
| AVALIDE
irbesartan and hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| AVALIDE
irbesartan and hydrochlorothiazide tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Chimie | 262699775 | API MANUFACTURE(0024-5855, 0024-5856) , ANALYSIS(0024-5855, 0024-5856) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EUROAPI Hungary Ltd. | 402388457 | ANALYSIS(0024-5855, 0024-5856) , API MANUFACTURE(0024-5855, 0024-5856) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc | 069777290 | ANALYSIS(0024-5855, 0024-5856) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CAMBREX PROFARMACO MILANO S.R.L. | 438051401 | ANALYSIS(0024-5855, 0024-5856) , API MANUFACTURE(0024-5855, 0024-5856) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Winthrop Industrie | 763683216 | ANALYSIS(0024-5855, 0024-5856) , MANUFACTURE(0024-5855, 0024-5856) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Winthrop Industrie | 571879985 | PACK(0024-5855, 0024-5856) , LABEL(0024-5855, 0024-5856) | |






