Drug Detail:Baxdela (Delafloxacin (oral/injection) [ del-a-flox-a-sin ])
Drug Class: Quinolones and fluoroquinolones
Highlights of Prescribing Information
BAXDELA (delafloxacin) tablets, for oral use
BAXDELA (delafloxacin) for injection, for intravenous use
Initial U.S. Approval: 2017
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS, and EXACERBATION OF MYASTHENIA GRAVIS
See full prescribing information for complete boxed warning.
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together (5.1), including:
- Tendinitis and tendon rupture (5.2)
- Peripheral neuropathy (5.3)
- Central nervous system effects (5.4)
Discontinue BAXDELA immediately and avoid the use of fluoroquinolones, including BAXDELA, in patients who experience any of these serious adverse reactions. (5.1)
- Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid BAXDELA in patients with known history of myasthenia gravis. (5.5)
Indications and Usage for Baxdela
BAXDELA is a fluoroquinolone antibacterial indicated for the treatment of adults with the following infections caused by designated susceptible bacteria:
- Acute Bacterial Skin and Skin Structure Infections (ABSSSI) (1.1)
- Community-Acquired Bacterial Pneumonia (CABP) (1.2)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of BAXDELA and other antibacterial drugs, BAXDELA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.3)
Baxdela Dosage and Administration
- For ABSSSI and CABP: Administer BAXDELA for injection 300 mg by intravenous infusion over 60 minutes, every 12 hours, or a 450 mg BAXDELA tablet orally every 12 hours. (2.1, 2.2)
- Recommended duration of treatment: (2.2)
- ABSSSI: 5 to 14 days
- CABP: 5 to 10 days
- Dosage for patients with renal impairment is based on the estimated glomerular filtration rate (eGFR) (2.3)
| Estimated Glomerular Filtration Rate (eGFR)(mL/min/1.73m2)* | Recommended Dosage Regimen for BAXDELA† | |
|---|---|---|
|
||
| Oral | Intravenous‡ | |
| 30-89 | No dosage adjustment | No dosage adjustment |
| 15-29 | No dosage adjustment | 200 mg every 12 hours |
| End Stage Renal Disease (ESRD) (< 15 including hemodialysis) | Not Recommended§ | |
Dosage Forms and Strengths
- For Injection: 300 mg of delafloxacin (equivalent to 433 mg delafloxacin meglumine) as a lyophilized powder in a single dose vial for reconstitution and further dilution before intravenous infusion. (3)
- Oral Tablets: 450 mg delafloxacin (equivalent to 649 mg delafloxacin meglumine). (3)
Contraindications
Known hypersensitivity to BAXDELA or other fluoroquinolones. (4, 5.6)
Warnings and Precautions
- Hypersensitivity Reactions: May occur after first or subsequent doses of BAXDELA. Discontinue BAXDELA at the first sign of a skin rash or any other sign of hypersensitivity. (5.6)
- Clostridium difficile-associated diarrhea: Evaluate if diarrhea occurs. (5.7)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 2%) are nausea, diarrhea, headache, transaminase elevations, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Melinta Therapeutics at 1-844-633-6568 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Use In Specific Populations
Renal Impairment: Closely monitor serum creatinine levels in patients with severe renal impairment (eGFR 15-29 mL/min/1.73 m2) receiving intravenous delafloxacin. If serum creatinine level increases occur, consider changing to oral delafloxacin. Discontinue BAXDELA if eGFR decreases to < 15 mL/min/1.73 m2 (8.6).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2021
Related/similar drugs
amoxicillin, doxycycline, ciprofloxacin, metronidazole, azithromycin, clindamycin, AugmentinFull Prescribing Information
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together (5.1), including:
- Tendinitis and tendon rupture (5.2)
- Peripheral neuropathy (5.3)
- Central nervous system effects (5.4)
Discontinue BAXDELA immediately and avoid the use of fluoroquinolones, including BAXDELA, in patients who experience any of these serious adverse reactions (5.1)
Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid BAXDELA in patients with known history of myasthenia gravis. (5.5)
1. Indications and Usage for Baxdela
1.1 Acute Bacterial Skin and Skin Structure Infections
BAXDELA is indicated in adults for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by the following susceptible microorganisms: Staphylococcus aureus (including methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), Staphylococcus haemolyticus, Staphylococcus lugdunensis, Streptococcus agalactiae, Streptococcus anginosus Group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus), Streptococcus pyogenes, Enterococcus faecalis, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
1.2 Community-Acquired Bacterial Pneumonia
BAXDELA is indicated in adults for the treatment of community-acquired bacterial pneumonia (CABP) caused by the following susceptible microorganisms: Streptococcus pneumoniae, Staphylococcus aureus (methicillin-susceptible [MSSA] isolates only), Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Haemophilus influenzae, Haemophilus parainfluenzae, Chlamydia pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae.
1.3 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of BAXDELA and other antibacterial drugs, BAXDELA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. Baxdela Dosage and Administration
2.2 Recommended Dosage Regimen
For treatment of adults with ABSSSI or CABP, the recommended dosage regimen of BAXDELA is described in Table 1 below.
| Infection | Dosage and Route of Administration | Total Duration (days) |
|---|---|---|
| ABSSSI |
| 5 to 14 |
| CABP | 5 to 10 |
2.3 Dosage in Patients with Renal Impairment
Table 2 below describes the dosage modification based on the estimated glomerular filtration rate (eGFR) that is recommended in patients with renal impairment. Dosage adjustment is required for patients with severe renal impairment (eGFR 15-29 mL/min/1.73m2).
In patients with severe renal impairment receiving BAXDELA intravenously, closely monitor serum creatinine levels and eGFR [see Use in Specific Populations (8.7)]. If serum creatinine level increases, consider switching to BAXDELA Tablets. Discontinue BAXDELA if eGFR decreases to < 15 mL/min/1.73 m2.
| Estimated Glomerular Filtration Rate (eGFR) (mL/min/1.73 m2)* | Recommended Dosage Regimen† | |
|---|---|---|
| BAXDELA Tablets | BAXDELA for Injection‡ | |
|
||
| 30-89 | No dosage adjustment | No dosage adjustment |
| 15-29 | No dosage adjustment | 200 mg every 12 hours Or 200 mg every 12 hours, then switch to a 450 mg BAXDELA tablet orally every 12 hours at the discretion of the physician |
| End Stage Renal Disease (ESRD) (< 15), including patients on hemodialysis (HD) | Not Recommended§ | |
4. Contraindications
BAXDELA is contraindicated in patients with known hypersensitivity to delafloxacin or any of the fluoroquinolone class of antibacterial drugs, or any of the components of BAXDELA [see Warnings and Precautions (5.6)].
5. Warnings and Precautions
5.1 Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy and Central Nervous System Effects
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions could occur within hours to weeks after starting a fluoroquinolone. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [see Warnings and Precautions (5.2, 5.3 and 5.4)].
Discontinue BAXDELA immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including BAXDELA, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
5.2 Tendinitis and Tendon Rupture
Fluoroquinolones have been associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and has also been reported with the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendons. Tendinitis or tendon rupture can occur, within hours or days of starting a fluoroquinolone, or as long as several months after completion of fluoroquinolone therapy. Tendinitis and tendon rupture can occur bilaterally.
This risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over age 60 years of age, in patients taking corticosteroid drugs, and, in patients with kidney, heart, and lung transplant. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors.
Discontinue BAXDELA immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon. Advise patients, at the first sign of tendon pain, swelling, or inflammation, to stop taking BAXDELA, to avoid exercise and use of the affected area, and to promptly contact their healthcare provider about changing to a non-quinolone antimicrobial drug. Avoid BAXDELA in patients who have a history of tendon disorders or have experienced tendinitis or tendon rupture.
5.3 Peripheral Neuropathy
Fluoroquinolones have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias, and weakness have been reported in patients receiving fluoroquinolones, including BAXDELA. Symptoms may occur soon after initiation of fluoroquinolones and may be irreversible in some patients [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Discontinue BAXDELA immediately if the patient experiences symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation and/or motor strength in order to minimize the development of an irreversible condition. Avoid fluoroquinolones, including BAXDELA in patients who have previously experienced peripheral neuropathy [see Adverse Reactions (6.1)].
5.5 Exacerbation of Myasthenia Gravis
Fluoroquinolones have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Post-marketing serious adverse reactions, including death and requirement for ventilator support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid BAXDELA in patients with known history of myasthenia gravis [see Patient Counseling Information (17)].
5.6 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving fluoroquinolone therapy. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria, and itching. Hypersensitivity reactions have been reported in patients receiving BAXDELA. These reactions may occur after first or subsequent doses of BAXDELA [see Adverse Reactions (6.1)]. Discontinue BAXDELA at the first appearance of a skin rash or any other sign of hypersensitivity.
5.7 Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported in users of nearly all systemic antibacterial drugs, including BAXDELA, with severity ranging from mild diarrhea to fatal colitis. Treatment with antibacterial agents can alter the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile should be discontinued, if possible. Appropriate measures such as fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.8 Risk of Aortic Aneurysm and Dissection
Epidemiologic studies report an increased risk of aortic aneurysm and dissection within two months following use of fluoroquinolones, particularly in elderly patients. The cause for the increased risk has not been identified. In patients with a known aortic aneurysm or patients who are at greater risk for aortic aneurysms, reserve BAXDELA for use only when there are no alternative antibacterial treatments available.
5.9 Development of Drug-Resistant Bacteria
Prescribing BAXDELA in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.10 Blood Glucose Disturbances
Fluoroquinolones have been associated with disturbances of blood glucose, including symptomatic hyperglycemia and hypoglycemia, usually in diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (e.g., glyburide) or with insulin. In these patients, careful monitoring of blood glucose is recommended. Severe cases of hypoglycemia resulting in coma or death have been reported with other fluoroquinolones. If a hypoglycemic reaction occurs in a patient being treated with BAXDELA, discontinue BAXDELA and initiate appropriate therapy immediately [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
- Disabling and Potentially Irreversible Serious Adverse Reactions [see Warnings and Precautions (5.1)]
- Tendinitis and Tendon Rupture [see Warnings and Precautions (5.2)]
- Peripheral Neuropathy [see Warnings and Precautions (5.3)]
- Central Nervous System Effects [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.6)]
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.7)]
- Blood Glucose Disturbances [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of BAXDELA cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
7. Drug Interactions
7.1 Chelation Agents: Antacids, Sucralfate, Metal Cations, Multivitamins
Fluoroquinolones form chelates with alkaline earth and transition metal cations. Oral administration of BAXDELA with antacids containing aluminum or magnesium, with sucralfate, with metal cations such as iron, or with multivitamins containing iron or zinc, or with formulations containing divalent and trivalent cations such as didanosine buffered tablets for oral suspension or the pediatric powder for oral solution, may substantially interfere with the absorption of BAXDELA, resulting in systemic concentrations considerably lower than desired. Therefore, BAXDELA should be taken at least 2 hours before or 6 hours after these agents [see Dosage and Administration (2.1)].
There are no data concerning an interaction of intravenous BAXDELA with oral antacids, sucralfate, multivitamins, didanosine, or metal cations. However, BAXDELA should not be co-administered with any solution containing multivalent cations, e.g., magnesium, through the same intravenous line [see Dosage and Administration (2.1)].
8. Use In Specific Populations
8.2 Lactation
Data
After single oral dose of 20 mg/kg (approximately 194 mg for a 60 kg patient) 14C-labeled delafloxacin on post-natal day 11, the radioactivity was transferred into the milk of lactating rats. The mean milk/plasma radioactivity concentration ratios in dams at 4 and 8 hours after dosing were 8.5 and 4.0, respectively, and essentially background by 24 hours. The rate of elimination of radioactivity was similar in milk and plasma. Absorption of radioactive drug by rat pups following nursing was observed.
8.4 Pediatric Use
Use in patients under 18 years of age is not recommended. Safety and effectiveness in pediatric patients below the age of 18 years have not been established. Pediatric studies were not conducted because risk-benefit considerations do not support the use of BAXDELA for ABSSSI in this population. Fluoroquinolones cause arthropathy in juvenile animals.
8.5 Geriatric Use
Of the 754 adult ABSSSI patients treated with BAXDELA, in Trials 1 and 2, 111/754 (15%) were 65 years of age and older. The clinical response rates at 48-72 hours for the BAXDELA-treated and comparator-treated patients were 84/111 (75.7%) and 72/101 (71.3%), respectively in ABSSSI patients aged 65 years and older compared to patients aged less than 65 years of age 529/643 (82.3%) and 538/655 (82.1%), respectively. In the safety population, of the 741 adult patients treated with BAXDELA, 18/110 (16.4%) patients aged 65 years and older and 146/631 (23.1%) patients aged less than 65 years had at least one adverse drug reaction.
Of the 431 adult CABP patients treated with BAXDELA, in Trial 3, 203/431 (47.1%) were 65 years of age and older, while 85/431 (19.7%) were 75 and over. The clinical response rates at 72-120 hours for the BAXDELA-treated and moxifloxacin-treated patients were 177/203 (87.2%) and 161/179 (89.9%), respectively in the CABP patients aged 65 years and older compared to patients aged less than 65 years old (206/228 (90.4%) and 220/249 (88.4%), respectively). In the safety population, of the 429 adult patients treated with BAXDELA, 10/84 (11.9%) patients aged 75 and older, 27/202 (13.4%) patients aged 65 years and older and 38/227 (16.7%) patients aged less than 65 years old had at least one adverse drug reaction.
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing BAXDELA to elderly patients especially those on corticosteroids. Patients should be informed of this potential adverse reaction and advised to discontinue BAXDELA and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur [see Warnings and Precautions (5.1)].
Epidemiologic studies report an increased rate of aortic aneurysm and dissection within two months following use of fluoroquinolones, particularly in elderly patients [see Warnings and Precautions (5.8)].
In elderly subjects (≥ 65 years), the mean Cmax and AUC∞ of delafloxacin were about 35% higher compared with young adults, which is not considered clinically significant [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
No dosage adjustment is necessary for BAXDELA in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment of BAXDELA is necessary in patients with mild (eGFR 60-89 mL/min/1.73 m2) or moderate (eGFR 30-59 mL/min/1.73 m2) renal impairment. The dose of BAXDELA intravenous IV infusion in patients with severe renal impairment (eGFR 15-29 mL/min/1.73 m2) should be decreased to 200 mg intravenously every 12 hours; the dose of oral BAXDELA in patients with severe renal impairment (eGFR 15-29 mL/min/1.73 m2) is 450 mg orally every 12 hours. BAXDELA is not recommended in patients with End Stage Renal Disease [ESRD] (eGFR of < 15 mL/min/1.73 m2) [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
In patients with severe renal impairment or ESRD (eGFR of < 15 mL/min/1.73 m2), accumulation of the intravenous vehicle, sulfobutylether-β-cyclodextrin (SBECD) occurs. Serum creatinine levels should be closely monitored in patients with severe renal impairment receiving intravenous BAXDELA. If serum creatinine level increases occur, consideration should be given to changing to oral BAXDELA. If eGFR decreases to < 15 mL/min/1.73 m2, BAXDELA should be discontinued.
10. Overdosage
Treatment of overdose with BAXDELA should consist of observation and general supportive measures. Hemodialysis removed about 19% of delafloxacin and 56% of SBECD (Sulfobutylether β cyclodextrin) after intravenous administration of BAXDELA [see Clinical Pharmacology (12.3)].
11. Baxdela Description
BAXDELA (delafloxacin) for Injection and BAXDELA (delafloxacin) Tablets contain meglumine salt of delafloxacin, a fluoroquinolone antibacterial. Delafloxacin meglumine is identified chemically as 1-Deoxy-1-(methylamino)-D-glucitol, 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (salt), the chemical structure of which is shown below. The meglumine salt has a molecular weight of 635.97 g/mol, whereas the molecular weight of the delafloxacin free acid is 440.76 g/mol.
Figure 1 Chemical Structure
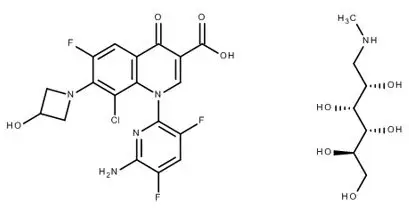
C18H12ClF3N4O4 ∙ C7H17NO5 M.W. 635.97
BAXDELA is intended for intravenous infusion or oral administration. BAXDELA is supplied as a sterile, lyophilized powder for injection and oral tablets as follows:
12. Baxdela - Clinical Pharmacology
12.2 Pharmacodynamics
The antibacterial activity of delafloxacin appears to best correlate with the ratio of area under the concentration-time curve of free delafloxacin to minimal inhibitory concentration (fAUC/MIC) for Gram-positive organisms such as Staphylococcus aureus and Gram-negative organisms such as Escherichia coli based on animal models of infection.
12.3 Pharmacokinetics
The pharmacokinetic parameters of delafloxacin following single- and multiple-dose (every 12 hours) oral (450 mg) and intravenous (300 mg) administration are shown in Table 6. Steady-state was achieved within approximately three days with accumulation of approximately 10% and 36% following IV and oral administration, respectively.
| Parameters | Tablet | Intravenous Injection | ||
|---|---|---|---|---|
| Single Dose 450 mg | Steady State 450 mg Q12h* | Single Dose 300 mg | Steady State 300 mg Q12h* |
|
| Cmax = maximum concentration; Tmax = time to reach Cmax; AUC = area under the concentration-time curve; CL = systemic clearance; CL/F = apparent oral clearance; Rac = accumulation ratio | ||||
|
||||
| Tmax (h)† | 0.75 (0.5, 4.0) | 1.00 (0.50, 6.00) | 1.0 (1.0, 1.2) | 1.0 (1.0, 1.0) |
| Cmax (µg/mL) | 7.17 (2.01) | 7.45 (3.16) | 8.94 (2.54) | 9.29 (1.83) |
| AUC (µg∙h/mL)‡ | 22.7 (6.21) | 30.8 (11.4) | 21.8 (4.54) | 23.4 (6.90) |
| CL or CL/F(L/h)§ | 20.6 (6.07) | 16.8 (6.54) | 14.1 (2.81) | 13.8 (3.96) |
| CLr (L/h) | - | - | 5.89 (1.53) | 6.69 (2.19) |
| Rac | - | 1.36 | - | 1.1 |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies have not been conducted with BAXDELA.
Delafloxacin was not mutagenic in a bacterial reverse mutation (Ames) assay, and was not clastogenic in a mouse bone marrow micronucleus test at ≥ 15 times the estimated human plasma exposure based on AUC. In an in vitro clastogenicity assay using isolated human lymphocytes, delafloxacin was negative in short incubations (~3 hours) and, at high cytotoxic concentrations (> 1.0 mM), was positive in a long incubation (~19 hours).
Delafloxacin did not affect the fertility of male and female rats up to the highest intravenous dose tested (120 mg/kg/day); female rats were dosed 2 weeks prior to mating and through gestation day 7 and male rats were treated for 28 days prior to mating and beyond for a total of 58-59 days. AUC in male and female (non-pregnant and pregnant) rats at 120 mg/kg/day delafloxacin intravenous was estimated to be approximately 5 times the estimated human plasma exposure based on AUC in separate intravenous toxicology studies in rats, one of which was a 2-week study that used a different vehicle for delafloxacin than in the fertility study, and another was an 8-day study in nonpregnant and pregnant (gestation day 13) rats that used the same vehicle for delafloxacin as in the fertility study.
13.2 Animal Toxicology and/or Pharmacology
Fluoroquinolone antibacterials are associated with degenerative changes in articular cartilage and arthropathy in skeletally immature animals. In a toxicology study of the formulated tablet in dogs, the femoral head of one of three high dose (480 mg/kg/day) females had minimal focal degeneration of the superficial articular cartilage and a small focal cleft in the articular cartilage. No other joints were examined.
14. Clinical Studies
14.1 Acute Bacterial Skin and Skin Structure Infections
A total of 1510 adults with acute bacterial skin and skin structure infections (ABSSSI) were randomized in 2 multicenter, multinational, double-blind, double-dummy, non-inferiority trials. Trial 1 compared BAXDELA 300 mg via intravenous infusion every 12 hours to comparator. In Trial 2, patients received BAXDELA 300 mg via intravenous infusion every 12 hours for 6 doses then made a mandatory switch to oral BAXDELA 450 mg every 12 hours. In both studies, the comparator was the intravenous combination of vancomycin 15 mg/kg actual body weight and aztreonam. Aztreonam therapy was discontinued if no gram-negative pathogens were identified in the baseline cultures.
In Trial 1, 331 patients with ABSSSI were randomized to BAXDELA and 329 patients were randomized to vancomycin plus aztreonam. Patients in this trial had the following infections: cellulitis (39%), wound infection (35%), major cutaneous abscess (25%), and burn infection (1%). The overall mean surface area of the infected lesion as measured by digital planimetry was 307 cm2. The average age of patients was 46 years (range 18 to 94 years). Patients were predominately male (63%) and white (91%); 32% had BMI ≥ 30 kg/m2. The population studied in Trial 1 included a distribution of patients with associated comorbidities such as hypertension (21%), diabetes (9%), and renal impairment (16%; 0.2% with severe renal impairment or ESRD). Current or recent history of drug abuse, including IV drug abuse, was reported by 55% of patients. Bacteremia was documented at baseline in 2% of patients.
In Trial 2, 423 patients were randomized to BAXDELA and 427 patients were randomized to vancomycin plus aztreonam. Patients in this trial had the following infections: cellulitis (48%), wound infection (26%), major cutaneous abscess (25%), and burn infection (1%). The overall mean surface area of the infected lesion, as measured by digital planimetry, was 353 cm2. The average age of patients was 51 years (range 18 to 93 years). Patients were predominately male (63%) and white (83%); 50 % had a BMI ≥ 30 kg/m2. The population studied in Trial 2 included a distribution of patients with associated comorbidities such as hypertension (31%), diabetes (13%) and renal impairment (16%; 0.2% with severe renal impairment or ESRD). Current or recent history of drug abuse, including IV drug abuse, was reported by 30% of patients. Bacteremia was documented at baseline in 2% of patients.
In both trials, objective clinical response at 48 to 72 hours post initiation of treatment was defined as a 20% or greater decrease in lesion size as determined by digital planimetry of the leading edge of erythema. Table 7 summarizes the objective clinical response rates in both of these trials.
| CI = Confidence Interval; ITT = Intent To Treat and includes all randomized patients | |||
|
|||
| Trial | BAXDELA (300 mg IV) | Vancomycin 15 mg/kg + Aztreonam | Treatment Difference†
(2-sided 95% CI) |
| Trial 1 | |||
| Total N | 331 | 329 | |
| Responder, n (%) | 259 (78.2%) | 266 (80.9%) | -2.6 (-8.8, 3.6) |
| BAXDELA (300 mg IV and 450 mg oral) | Vancomycin 15 mg/kg + Aztreonam | ||
| Trial 2 | |||
| Total N | 423 | 427 | |
| Responder, n (%) | 354 (83.7%) | 344 (80.6%) | 3.1 (-2.0, 8.3) |
In both trials, an investigator assessment of response was made at Follow-up (Day 14 ± 1) in the ITT and CE populations. Success was defined as "cure + improved," where patients had complete or near resolution of signs and symptoms, with no further antibacterial needed. The success rates in the ITT and CE populations are shown in Table 8.
| CI = Confidence Interval; ITT = Intent To Treat and includes all randomized patients; CE = Clinically Evaluable consisted of all ITT patients who had a diagnosis of ABSSSI, received at least 80% of expected doses of study drug, did not have any protocol deviations that would affect the assessment of efficacy and had investigator assessment at the Follow-Up Visit. | |||
|
|||
| Trial | BAXDELA (300 mg IV) | Vancomycin 15 mg/kg + Aztreonam | Treatment Difference*
(2-sided 95% CI) |
| Trial 1 | |||
| Success†, n/N (%) ITT | 270/331 (81.6%) | 274/329 (83.3%) | -1.7 (-7.6, 4.1) |
| Success†, n/N (%) CE | 232/240 (96.7%) | 238/244 (97.5%) | -0.9 (-4.3, 2.4) |
| BAXDELA (300 mg IV and 450 mg Oral) | Vancomycin 15 mg/kg + Aztreonam | ||
| Trial 2 | |||
| Success, n/N (%) ITT | 369/423 (87.2%) | 362/427 (84.8%) | 2.5 (-2.2, 7.2) |
| Success, n/N (%) CE | 339/353 (96.0%) | 319/329 (97.0%) | -0.9 (-3.9, 2.0) |
Six delafloxacin patients had baseline S. aureus bacteremia with ABSSSI. Five of these 6 patients (83.3%) were clinical responders at 48 to 72 hours and 5/6 (83.3%) were considered clinical success for ABSSSI at Day 14 ± 1. Two delafloxacin patients had baseline Gram-negative bacteremia (K. pneumoniae and P. aeruginosa), and both were clinical responders and successes.
The investigator assessments of clinical success rates were also similar between treatment groups at Late Follow-up (LFU, day 21-28).
Objective clinical response and investigator-assessed success by baseline pathogens from the primary infection site or blood cultures for the microbiological ITT (MITT) patient population pooled across Trial 1 and Trial 2 are presented in Table 9.
| Clinical Response† at 48–72 hours | Investigator-Assessed Success‡ at Follow-up | |||
|---|---|---|---|---|
| BAXDELA | Comparator | BAXDELA | Comparator | |
| Pathogen | n/N (%) | n/N (%) | n/N (%) | n/N (%) |
|
||||
| Staphylococcus aureus | 271/319 (85.0) | 269/324 (83.0) | 275/319 (86.2) | 269/324 (83.0) |
| Methicillin-susceptible§ | 149/177 (84.2) | 148/183 (80.9) | 154/177 (87.0) | 153/183 (83.6) |
| Methicillin-resistant§ | 125/144 (86.8) | 121/141 (85.8) | 122/144 (84.7) | 116/141 (82.3) |
| Streptococcus pyogenes | 17/23 (73.9) | 9/18 (50.0) | 21/23 (91.3) | 16/18 (88.9) |
| Staphylococcus haemolyticus | 11/15 (73.3) | 7/8 (87.5) | 13/15 (86.7) | 7/8 (87.5) |
| Streptococcus agalactiae | 10/14 (71.4) | 9/12 (75.0) | 12/14 (85.7) | 11/12 (91.7) |
| Streptococcus anginosus Group | 59/64 (92.2) | 55/61 (90.2) | 54/64 (84.4) | 47/61 (77.0) |
| Staphylococcus lugdunensis | 8/11 (72.7) | 6/9 (66.7) | 10/11 (90.9) | 8/9 (88.9) |
| Enterococcus faecalis | 11/11 (100.0) | 12/16 (75.0) | 9/11 (81.8) | 14/16 (87.5) |
| Escherichia coli | 12/14 (85.7) | 16/20 (80.0) | 12/14 (85.7) | 18/20 (90.0) |
| Enterobacter cloacae | 10/14 (71.4) | 8/11 (72.7) | 12/14 (85.7) | 10/11 (90.9) |
| Klebsiella pneumoniae | 19/22 (86.4) | 22/23 (95.7) | 20/22 (90.9) | 21/23 (91.3) |
| Pseudomonas aeruginosa | 9/11 (81.8) | 11/12 (91.7) | 11/11 (100.0) | 12/12 (100.0) |
14.2 Community-Acquired Bacterial Pneumonia
A total of 859 adults with CABP were randomized in a multicenter, multinational, double-blind, double-dummy, noninferiority trial comparing BAXDELA to moxifloxacin (Trial 3, NCT 02679573). In this trial, BAXDELA for injection 300 mg was administered intravenously (IV) every 12 hours with an option to switch to BAXDELA tablet 450 mg orally every 12 hours. Moxifloxacin 400 mg was administered IV every 24 hours with an option to switch to moxifloxacin tablet 400 mg orally every 24 hours. Switch to oral treatment was allowed after a minimum of 3 days of IV dosing. Total treatment duration was 5 to 10 days. In the moxifloxacin arm, the investigator could switch patients to linezolid 600 mg every 12 hours if methicillin-resistant Staphylococcus aureus (MRSA) was confirmed.
A total of 431 patients were randomized to BAXDELA and 428 to moxifloxacin. Patient demographic and baseline characteristics were balanced between the treatment arms. In this trial, 12.9% of patients were in PORT Risk Class II, 60.3% were in PORT Risk Class III, 25.4% were in PORT Risk Class IV, and 1.4% were in PORT Risk Class V. The average age of patients was 60 years (range 18 to 93 years). Patients were predominantly male (58.7%) and white (91.5%); average BMI was 26.9 kg/m2. Associated comorbidities included pre-existing pulmonary disease (13.6%), cardiac disease (23.9%), diabetes (15.3%), and mild to severe renal impairment (76.9%). Bacteremia was documented at baseline in 1.5% of patients. The majority of sites were in Eastern Europe, which accounted for 82.8% of enrollment. One subject (0.2%) was enrolled in the BAXDELA arm and 5 (1.2%) in the moxifloxacin arm from the United States.
Early clinical response (ECR) at 72-120 hours after the first dose was defined as survival with improvement in at least two of four symptoms (cough, sputum production, chest pain, dyspnea) from baseline without deterioration in any of these symptoms, and without use of additional antimicrobial therapy for treatment of the current CABP infection due to lack of efficacy.
| Trial 3 | BAXDELA (300 mg IV and 450 mg oral) | Moxifloxacin (400 mg IV and 400 mg oral) | Treatment Difference†
(2-sided 95% CI) |
|---|---|---|---|
| CI = Confidence Interval; ITT = Intent To Treat includes all randomized patients | |||
|
|||
| Total N | 431 | 428 | |
| Responder n (%) | 383 (88.9) | 381 (89.0) | -0.2 (-4.4, 4.1) |
Clinical response was also assessed by the investigator at the test of cure (TOC) visit and defined as survival with resolution or near resolution of the symptoms of CABP present at study entry, and no use of additional antimicrobial therapy for the current CABP infection, and no new symptoms associated with the current CABP infection.
Clinical response rates at the TOC visit for the ITT and Clinically Evaluable (CE) populations are presented in Table 11.
| Trial 3 | BAXDELA (300 mg IV and 450 mg oral) | Moxifloxacin (400 mg IV and 400 mg oral) | Treatment Difference*
(2-sided 95% CI) |
|---|---|---|---|
| CI = Confidence Interval; ITT = Intent To Treat and includes all randomized patients; CE = Clinically Evaluable | |||
| Clinically Evaluable consisted of all ITT patients who had evidence of acute CABP, received at least 80% of expected doses of the correct study drug, did not receive any concomitant, systemic antibacterial therapy except for lack of efficacy, and did not have any protocol deviations that would affect the assessment of efficacy. | |||
|
|||
| Success†, n/N (%) ITT | 390/431 (90.5) | 384/428 (89.7) | 0.8 (-3.3, 4.8) |
| Success†, n/N (%) CE | 376/397 (94.7) | 373/394 (94.7) | 0.0 (-3.2, 3.3) |
Early clinical response and investigator-assessed clinical response at the TOC visit is presented in Table 12 by baseline pathogen for the Microbiological ITT (MITT) population which comprised all randomized patients who had a baseline pathogen identified that is known to cause CABP.
| Early Clinical Response† at 96 hours ± 24 hours | Investigator-Assessed Success‡ at Test-of Cure (TOC) | |||
|---|---|---|---|---|
| BAXDELA | Moxifloxacin | BAXDELA | Moxifloxacin | |
| Pathogen | n/N (%) | n/N (%) | n/N (%) | n/N (%) |
|
||||
| Staphylococcus aureus | 24/26 (92.3) | 25/28 (89.3) | 24/26 (92.3) | 26/28 (92.9) |
| Methicillin-susceptible | 22/24 (91.7) | 25/28 (89.3) | 22/24 (91.7) | 26/28 (92.9) |
| Streptococcus pneumoniae | 66/71 (93.0) | 51/62 (82.3) | 64/71 (90.1) | 54/62 (87.1) |
| Haemophilus influenzae | 25/26 (96.2) | 31/35 (88.6) | 24/26 (92.3) | 31/35 (88.6) |
| Haemophilus parainfluenzae | 30/32 (93.8) | 27/33 (81.8) | 30/32 (93.8) | 26/33 (78.8) |
| Escherichia coli | 15/16 (93.8) | 8/11 (72.7) | 15/16 (93.8) | 10/11 (90.9) |
| Klebsiella pneumoniae | 13/17 (76.5) | 15/16 (93.8) | 14/17 (82.4) | 16/16 (100.0) |
| Pseudomonas aeruginosa | 12/13 (92.3) | 10/11 (90.9) | 11/13 (84.6) | 11/11 (100.0) |
| Chlamydia pneumoniae | 24/25 (96.0) | 14/16 (87.5) | 25/25 (100.0) | 16/16 (100.0) |
| Legionella pneumophilia | 27/29 (93.1) | 28/33 (84.8) | 27/29 (93.1) | 32/33 (97.0) |
| Mycoplasma pneumoniae | 30/35 (85.7) | 29/30 (96.7) | 34/35 (97.1) | 30/30 (100.0) |
16. How is Baxdela supplied
16.1 BAXDELA for Injection
BAXDELA is supplied as a sterile, lyophilized powder in single-dose clear glass vials of 300 mg delafloxacin (equivalent to 433 mg delafloxacin meglumine). The lyophilized powder is a light yellow to tan cake, which may exhibit cracking and shrinkage and slight variation in texture and color.
They are supplied as follows: 300 mg single-dose vials (NDC 70842-102-01), packaged in cartons of 10 vials (NDC 70842-102-03).
16.2 BAXDELA Tablets
BAXDELA Tablets contain 450 mg delafloxacin (equivalent to 649 mg delafloxacin meglumine); each modified capsule-shaped tablet in beige to mottled beige color is debossed with RX3341 on one side. They are supplied as follows:
Bottles of 20 tablets with child-resistant closure (NDC 70842-101-01)
Unit dose blister packs which contain 20 tablets (2 blister cards of 10 tablets each)
(20 tablet blister pack: NDC 70842-101-02, 10 tablet blister card: NDC 70842-101-03)
16.3 Storage and Handling
BAXDELA Tablets and BAXDELA for Injection should be stored at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
The reconstituted powder may be stored for up to 24 hours under refrigerated or controlled room temperature and then further diluted for intravenous infusion. The reconstituted solution in the infusion bag may be stored under refrigerated or controlled room temperature conditions for up to 24 hours [see Dosage and Administration (2.4)]. Do not freeze.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration MEL011-R007 | Revised: 6/2021 | ||
| MEDICATION GUIDE BAXDELA® (Bax-de'-lah) (delafloxacin) for injection |
|||
| BAXDELA® (Bax-de'-lah) (delafloxacin) tablets for oral use |
|||
| What is the most important information I should know about BAXDELA? | |||
| BAXDELA, a fluoroquinolone antibacterial medicine, can cause serious side effects. Some of these serious side effects can happen at the same time and could result in death. If you get any of the following serious side effects while you take BAXDELA, you should stop taking BAXDELA immediately and get medical help right away. | |||
|
|||
|
|
||
|
|||
|
|||
|
|
||
|
|||
| What is BAXDELA? | |||
BAXDELA is a fluoroquinolone antibacterial medicine used to treat certain types of infections caused by certain germs called bacteria in adults 18 years or older. These bacterial infections include:
|
|||
| It is not known if BAXDELA is safe and effective in people under 18 years of age, and use in people under 18 years of age is not recommended. Children younger than 18 years of age may have a higher chance of getting bone, joint, and tendon (musculoskeletal) problems while taking fluoroquinolone antibacterial medicines. | |||
| Sometimes infections are caused by viruses rather than by bacteria. Examples include viral infections in the sinuses and lungs, such as the common cold or flu. Antibacterial medicines, including BAXDELA, do not kill viruses. Call your healthcare provider if you think your condition is not getting better while you are taking BAXDELA. | |||
| Do not take BAXDELA if: | |||
| you have ever had a severe allergic reaction to an antibacterial known as a fluoroquinolone, or if you are allergic to any of the ingredients in BAXDELA. Ask your healthcare provider if you are not sure. See the list of ingredients in BAXDELA at the end of this Medication Guide. | |||
| What should I tell my healthcare provider before taking BAXDELA? | |||
| See "What is the most important information I should know about BAXDELA?" | |||
Before you take BAXDELA, tell your healthcare provider about all your medical conditions, including if you:
|
|||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal and dietary supplements. | |||
BAXDELA and other medicines can affect each other causing side effects. Especially tell your healthcare provider if you take:
|
|||
| Ask your healthcare provider if you are not sure if any of your medicines are listed above. Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. | |||
How should I take BAXDELA?
|
|||
| What should I avoid while taking BAXDELA? | |||
| BAXDELA can make you feel dizzy and lightheaded. Do not drive, operate machinery, or do other activities that require mental alertness or coordination until you know how BAXDELA affects you. | |||
| What are the possible side effects of BAXDELA? | |||
BAXDELA may cause serious side effects, including:
|
|||
|
|
||
| Skin rash may happen in people taking fluoroquinolones even after only 1 dose. Stop taking BAXDELA at the first sign of a skin rash and call your healthcare provider. Skin rash may be a sign of a more serious reaction to BAXDELA. | |||
|
|||
| The most common side effects of BAXDELA include: | |||
|
|
||
| These are not all the possible side effects of BAXDELA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
| How should I store BAXDELA? | |||
BAXDELA Tablets:
|
|||
| Keep BAXDELA and all medicines out of the reach of children. | |||
| General information about the safe and effective use of BAXDELA. | |||
| Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BAXDELA for a condition for which it was not prescribed. Do not give BAXDELA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BAXDELA that is written for health professionals. | |||
What are the ingredients in BAXDELA?
|
|||
| Distributed by: Melinta Therapeutics, LLC 300 Tri-State International, Lincolnshire, Illinois, 60069 USA Trademarks depicted herein are the property of their respective owners. ©2021 Melinta Therapeutics, LLC All rights reserved For more information go to BAXDELA.com or call 1-844-633-6568. |
|||
| BAXDELA
delafloxacin meglumine tablet |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| BAXDELA
delafloxacin meglumine injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Melinta Therapeutics, LLC (079949853) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Curia Indiana, LLC | 020593403 | ANALYSIS(70842-101, 70842-102) | |