Drug Detail:Tice bcg live (for intravesical use) (Bcg intravesical (bee cee jee))
Drug Class: Miscellaneous antineoplastics
Adverse Reactions/Side Effects
Although BCG vaccination often causes local reactions, serious or long-term complications are rare.{3} Reactions that can be expected after vaccination include moderate axillary or cervical lymphadenopathy and induration and subsequent pustule formation at the injection site; these reactions can persist for as long as 3 months after vaccination. More serious local reactions include ulceration at the vaccination site, regional suppurative lymphadenitis with draining sinuses, and caseous lesions or purulent draining at the puncture site. These manifestations might occur up to 5 months after vaccination and could persist for several weeks. The intensity and duration of the local reaction depends on the depth of penetration of the multiple puncture device and individual variations in patients' tissue reactions. Slight tenderness at the puncture site may be encountered as well as some itching. The initial skin lesions usually appear within 10–14 days and consist of small red papules at the site. The papules reach maximum diameter (about 3 mm) after 4 to 6 weeks, after which they may scale and then slowly subside.
The most serious complication of BCG vaccination is disseminated BCG infection.{24,25} The most frequent disseminated infection is BCG osteomyelitis (0.01 to 43 cases per million doses of vaccine administered) which usually occurs 4 months to 2 years after vaccination. Fatal disseminated BCG infection has occurred at a rate of 0.06–1.56 cases per million doses; these deaths occurred primarily among immunocompromised persons.
BCG Vaccine Dosage and Administration
Treatment and Schedule
BCG vaccination is reserved for persons who have a reaction of less than 5mm induration after skin testing with 5 TU of PPD tuberculin. The preferred method of skin testing is the Mantoux tuberculin skin-test using 0.1 mL of 5 tuberculin units (TU) of PPD.{3} It is recommended that a Mantoux skin-test be performed prior to BCG vaccination to demonstrate the absence of tuberculous infection.
The vaccine is to be administered after fully explaining the risks and benefits to the vaccinee, parent, or guardian. BCG vaccination should not be given to individuals previously infected with M. tuberculosis. The vaccine is administered percutaneously utilizing a sterile, single-use multiple puncture device. The multiple puncture device consists of a plastic holder for a thin, wafer-like stainless steel plate from which 36 points protrude (Figure 1). After the vaccine is prepared, the skin site is cleansed with an alcohol or acetone sponge and allowed to dry thoroughly.
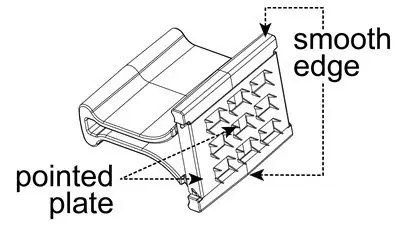
Figure 1
- 1.
- Administer the vaccine in the deltoid region (Figure 2). Position the arm to maintain a horizontal surface where the vaccine is to be placed.
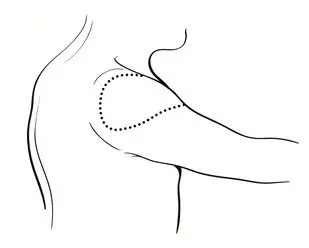
Figure 2
- 2.
- Drop the immunizing dose of 0.2–0.3 mL of BCG VACCINE from the syringe and needle onto the cleansed surface of the skin (Figure 3) and spread over a 1" by 2" area using the smooth edge of the multiple puncture device (Figure 4).
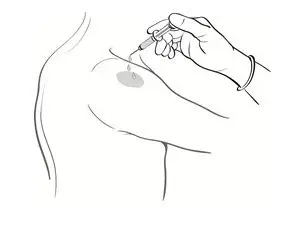
Figure 3
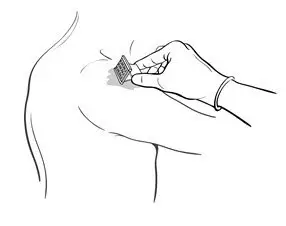
Figure 4
- 3.
- Grasp the arm firmly from underneath, tensing the skin. Center the multiple puncture device over the vaccine and apply firm downward pressure such that the device points are well buried in the skin (Figure 5).
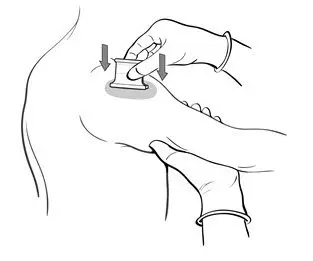
Figure 5
- 4.
- Maintain pressure for 5 seconds. Do not "rock" the device. Release the pressure underneath the arm and remove the device. In a successful procedure the points puncture the skin. If the points do not puncture the skin, the puncture procedure must be repeated.
- 5.
- After successful puncture, spread vaccine as evenly as possible over the puncture area with the smooth edge of the device (Figure 4). An additional 1–2 drops of BCG VACCINE may be added to ensure a very wet vaccination site.
- 6.
- Use the multiple puncture device once and discard in a standard biohazardous sharps container.
- 7.
- Loosely cover the site and keep dry for 24 hours.
- 8.
- Advise the patient that the vaccine contains live organisms. Although the vaccine will not survive in a dry state for long, infection of others is possible.
Tuberculin reactivity resulting from BCG vaccination should be documented. A vaccinated person should be tuberculin skin tested 2–3 months after BCG administration, and the test results, in millimeters of induration, should be recorded in the person's medical record.{9} Vaccination should be repeated for those who remain tuberculin negative to 5 TU of tuberculin after 2–3 months.
Pediatric Dose
Do not administer INTRAVENOUSLY, SUBCUTANEOUSLY, INTRAMUSCULARLY, OR INTRADERMALLY. Administer the vaccine in the deltoid region.
In infants less than 1 month old, the dosage of BCG VACCINE should be reduced by one-half, by using 2 mL of Sterile Water for Injection, USP at 4-25°C (39-77°F) when reconstituting. If a vaccinated infant remains tuberculin negative to 5 TU on skin testing, and if indications for vaccination persist, the infant should receive a full dose after 1 year of age.
| BCG VACCINE
bacillus calmette-guerin substrain tice live antigen injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |