Drug Detail:Belsomra (Suvorexant)
Drug Class: Miscellaneous anxiolytics, sedatives and hypnotics
Highlights of Prescribing Information
BELSOMRA® (suvorexant) tablets, for oral use, C-IV
Initial U.S. Approval: 2014
Indications and Usage for Belsomra
BELSOMRA is an orexin receptor antagonist indicated for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance (1).
Belsomra Dosage and Administration
- Use the lowest dose effective for the patient (2.1).
- Recommended dose is 10 mg, no more than once per night taken within 30 minutes of going to bed, with at least 7 hours remaining before the planned time of awakening. If the 10 mg dose is well-tolerated but not effective, the dose can be increased, not to exceed 20 mg once daily (2.1, 2.2).
- Time to effect may be delayed if taken with or soon after a meal (2.1).
Dosage Forms and Strengths
Tablets, 5 mg, 10 mg, 15 mg, 20 mg (3).
Contraindications
BELSOMRA is contraindicated in patients with narcolepsy (4).
Warnings and Precautions
- CNS Depressant Effects and Daytime Impairment: Risk of impaired alertness and motor coordination, including impaired driving; risk increases with dose; caution patients taking 20 mg against next-day driving and other activities requiring complete mental alertness (5.1).
- Worsening of Depression/Suicidal Ideation: Worsening of depression or suicidal thinking may occur. Risk increases with dose. Immediately evaluate any new behavioral changes (5.2).
- Complex Sleep Behaviors: Behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur. Discontinue immediately if a complex sleep behavior occurs (5.3).
- Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, and Cataplexy-like Symptoms: May occur with the use of BELSOMRA. Risk increases with dose (5.4).
- Compromised Respiratory Function: Effect on respiratory function should be considered (5.5, 8.6).
- Need to Evaluate for Co-morbid Diagnoses: Reevaluate if insomnia persists after 7 to 10 days of treatment (5.6).
Adverse Reactions/Side Effects
The most common adverse reaction (reported in 5% or more of patients treated with BELSOMRA and at least twice the placebo rate) with BELSOMRA was somnolence (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
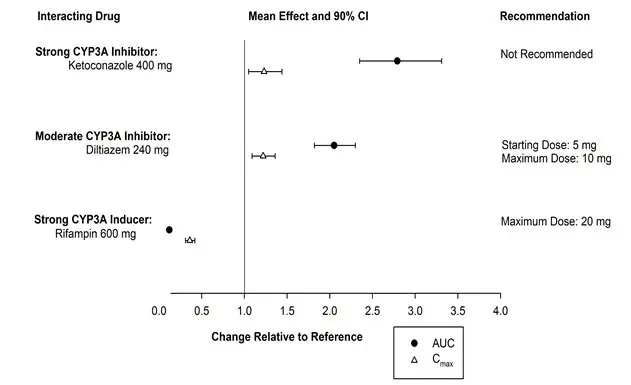
- CYP3A inhibitors: Recommended dose is 5 mg when used with moderate CYP3A inhibitors. Dose can be increased to 10 mg once per night if the 5 mg dose is not effective. Not recommended for use in patients taking strong CYP3A inhibitors (2.4, 7.2).
- Strong CYP3A inducers: Efficacy may be reduced (7.2).
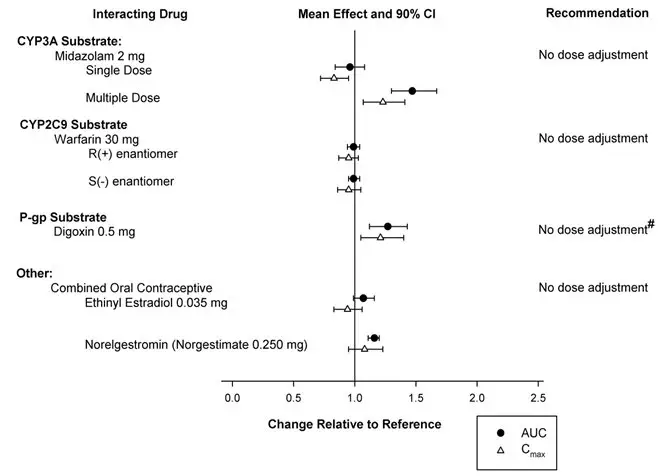
- Digoxin: Monitor digoxin concentrations (7.3).
Use In Specific Populations
Patients with severe hepatic impairment: Not recommended (8.7).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2023
Full Prescribing Information
1. Indications and Usage for Belsomra
BELSOMRA® (suvorexant) is indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance.
2. Belsomra Dosage and Administration
2.1 Dosing Information
Use the lowest dose effective for the patient. For all BELSOMRA doses, take no more than once per night within 30 minutes of going to bed (with at least 7 hours remaining prior to planned awakening). Time to effect of BELSOMRA may be delayed if taken with or soon after a meal [see Clinical Pharmacology (12.3)].
The recommended dose for BELSOMRA is 10 mg, taken no more than once per night. If the 10 mg dose is well-tolerated but not effective, the dose can be increased. The maximum recommended dose of BELSOMRA is 20 mg taken no more than once per night.
2.2 Special Populations
Exposure to BELSOMRA is increased in obese compared to non-obese patients, and in women compared to men. Particularly in obese women, the increased risk of exposure-related adverse effects should be considered before increasing the dose [see Clinical Pharmacology (12.3)].
2.3 Use with CNS Depressants
When BELSOMRA is combined with other CNS depressant drugs, dosage reduction of BELSOMRA and/or the other drug(s) may be necessary because of potentially additive effects [see Warnings and Precautions (5.1)].
2.4 Dosage Adjustments with CYP3A Inhibitors
When used with moderate CYP3A inhibitors, the recommended dosage of BELSOMRA is 5 mg taken no more than once per night (the dose generally should not exceed 10 mg). BELSOMRA is not recommended for use with strong CYP3A inhibitors [see Drug Interactions (7.2)].
3. Dosage Forms and Strengths
- 5 mg tablets are yellow, round, film-coated tablets with "5" on one side and plain on the other side.
- 10 mg tablets are green, round, film-coated tablets with "33" on one side and plain on the other side.
- 15 mg tablets are white, oval, film-coated tablets with the Merck logo on one side and "325" on the other side.
- 20 mg tablets are white, round, film-coated tablets with the Merck logo and "335" on one side and plain on the other side.
5. Warnings and Precautions
5.1 CNS Depressant Effects and Daytime Impairment
BELSOMRA is a central nervous system (CNS) depressant that can impair daytime wakefulness even when used as prescribed. Prescribers should monitor for somnolence and CNS depressant effects, but impairment can occur in the absence of symptoms, and may not be reliably detected by ordinary clinical exam (i.e., less than formal testing of daytime wakefulness and/or psychomotor performance). CNS depressant effects may persist in some patients for up to several days after discontinuing BELSOMRA.
BELSOMRA can impair driving skills and may increase the risk of falling asleep while driving. Discontinue or decrease the dose in patients who drive if daytime somnolence develops. In a study of healthy adults, driving ability was impaired in some individuals taking 20 mg BELSOMRA [see Clinical Studies (14.2)]. Although pharmacodynamic tolerance or adaptation to some adverse depressant effects of BELSOMRA may develop with daily use, patients using the 20 mg dose of BELSOMRA should be cautioned against next-day driving and other activities requiring full mental alertness. Patients taking lower doses of BELSOMRA should also be cautioned about the potential for driving impairment because there is individual variation in sensitivity to BELSOMRA.
Co-administration with other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol) increases the risk of CNS depression. Patients should be advised not to consume alcohol in combination with BELSOMRA because of additive effects [see Drug Interactions (7.1)]. Dosage adjustments of BELSOMRA and of concomitant CNS depressants may be necessary when administered together because of potentially additive effects. The use of BELSOMRA with other drugs to treat insomnia is not recommended [see Dosage and Administration (2.3)].
The risk of next-day impairment, including impaired driving, is increased if BELSOMRA is taken with less than a full night of sleep remaining, if a higher than the recommended dose is taken, if co-administered with other CNS depressants, or if co-administered with other drugs that increase blood levels of BELSOMRA. Patients should be cautioned against driving and other activities requiring complete mental alertness if BELSOMRA is taken in these circumstances.
Because BELSOMRA can cause drowsiness, patients, particularly the elderly, are at higher risk of falls.
5.2 Worsening of Depression/Suicidal Ideation
In clinical studies, a dose-dependent increase in suicidal ideation was observed in patients taking BELSOMRA as assessed by questionnaire. Immediately evaluate patients with suicidal ideation or any new behavioral sign or symptom.
In primarily depressed patients treated with sedative-hypnotics, worsening of depression, and suicidal thoughts and actions (including completed suicides) have been reported. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdose is more common in this group of patients; therefore, the lowest number of tablets that is feasible should be prescribed for the patient at any one time.
The emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
5.3 Complex Sleep Behaviors
Complex sleep behaviors, including sleep-walking, sleep-driving, and engaging in other activities while not fully awake (e.g., preparing and eating food, making phone calls, having sex), have been reported to occur with the use of hypnotics such as BELSOMRA. These events can occur in hypnotic-naïve as well as in hypnotic-experienced persons. Patients usually do not remember these events. Complex sleep behaviors may occur following the first or any subsequent use of BELSOMRA, with or without the concomitant use of alcohol and other CNS depressants [see Drug Interactions (7.1)]. Discontinue BELSOMRA immediately if a patient experiences a complex sleep behavior.
5.4 Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, Cataplexy-Like Symptoms
Sleep paralysis, an inability to move or speak for up to several minutes during sleep-wake transitions, and hypnagogic/hypnopompic hallucinations, including vivid and disturbing perceptions by the patient, can occur with the use of BELSOMRA. Prescribers should explain the nature of these events to patients when prescribing BELSOMRA.
Symptoms similar to mild cataplexy can occur, with risk increasing with the dose of BELSOMRA. Such symptoms can include periods of leg weakness lasting from seconds to a few minutes, can occur both at night and during the day, and may not be associated with an identified triggering event (e.g., laughter or surprise).
5.5 Patients with Compromised Respiratory Function
Effect of BELSOMRA on respiratory function should be considered if prescribed to patients with compromised respiratory function. BELSOMRA has not been studied in patients with severe obstructive sleep apnea (OSA) or severe chronic obstructive pulmonary disease (COPD) [see Use in Specific Populations (8.6)].
5.6 Need to Evaluate for Co-morbid Diagnoses
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, treatment of insomnia should be initiated only after careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new cognitive or behavioral abnormalities may be the result of an unrecognized underlying psychiatric or physical disorder and can emerge during the course of treatment with hypnotic drugs such as BELSOMRA.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections:
- CNS Depressant Effects and Daytime Impairment [see Warnings and Precautions (5.1)]
- Worsening of Depression/Suicidal Ideation [see Warnings and Precautions (5.2)]
- Complex Sleep Behaviors [see Warnings and Precautions (5.3)]
- Sleep Paralysis, Hypnagogic/Hypnopompic Hallucinations, Cataplexy-Like Symptoms [see Warnings and Precautions (5.4)]
- Patients with Compromised Respiratory Function [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In 3-month controlled efficacy trials (Study 1 and Study 2), 1263 patients were exposed to BELSOMRA including 493 patients who received BELSOMRA 15 mg or 20 mg (see Table 1).
In a long-term study, additional patients (n=521) were treated with BELSOMRA at higher than recommended doses, including a total of 160 patients who received BELSOMRA for at least one year.
| Patients Treated | BELSOMRA 15 mg | BELSOMRA 20 mg |
|---|---|---|
| For ≥ 1 Day (n) | 202 | 291 |
| Men (n) | 69 | 105 |
| Women (n) | 133 | 186 |
| Mean Age (years) | 70 | 45 |
| For ≥ 3 Months (n) | 118 | 172 |
The pooled safety data described below (see Table 2) reflect the adverse reaction profile during the first 3 months of treatment.
Most Common Adverse Reactions
In clinical trials of patients with insomnia treated with BELSOMRA 15 mg or 20 mg, the most common adverse reaction (reported in 5% or more of patients treated with BELSOMRA and at least twice the placebo rate) was somnolence (BELSOMRA 7%; placebo 3%).
Table 2 shows the percentage of patients with adverse reactions during the first three months of treatment, based on the pooled data from 3-month controlled efficacy trials (Study 1 and Study 2).
At doses of 15 or 20 mg, the incidence of somnolence was higher in females (8%) than in males (3%). Of the adverse reactions reported in Table 2, the following occurred in women at an incidence of at least twice that in men: headache, abnormal dreams, dry mouth, cough, and upper respiratory tract infection.
The adverse reaction profile in elderly patients was generally consistent with non-elderly patients. The adverse reactions reported during long-term treatment up to 1 year were generally consistent with those observed during the first 3 months of treatment.
| Placebo | BELSOMRA (20 mg in non-elderly or 15 mg in elderly patients) |
|
|---|---|---|
| n=767 | n=493 | |
| Gastrointestinal Disorders | ||
| Diarrhea | 1 | 2 |
| Dry mouth | 1 | 2 |
| Infections and Infestations | ||
| Upper respiratory tract infection | 1 | 2 |
| Nervous System Disorders | ||
| Headache | 6 | 7 |
| Somnolence | 3 | 7 |
| Dizziness | 2 | 3 |
| Psychiatric Disorders | ||
| Abnormal dreams | 1 | 2 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough | 1 | 2 |
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of BELSOMRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: palpitations, tachycardia
Gastrointestinal disorders: nausea, vomiting
Nervous system disorders: psychomotor hyperactivity
Psychiatric disorders: anxiety
Skin and subcutaneous tissue disorders: pruritus
7. Drug Interactions
7.1 CNS-Active Agents
When BELSOMRA was co-administered with alcohol, additive psychomotor impairment was demonstrated. There was no alteration in the pharmacokinetics of BELSOMRA [see Warnings and Precautions (5.1, 5.3) and Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.5 Geriatric Use
Of the total number of patients treated with BELSOMRA (n=1784) in controlled clinical safety and efficacy studies, 829 patients were 65 years and over, and 159 patients were 75 years and over. No clinically meaningful differences in safety or effectiveness were observed between these patients and younger patients at the recommended doses [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
Because BELSOMRA can increase drowsiness, patients, particularly the elderly, are at a higher risk of falls [see Warnings and Precautions (5.1)].
8.6 Patients with Compromised Respiratory Function
Effects of BELSOMRA on respiratory function should be considered if prescribed to patients with compromised respiratory function.
9. Drug Abuse and Dependence
9.2 Abuse
Abuse of BELSOMRA poses an increased risk of somnolence, daytime sleepiness, impaired reaction time and impaired driving skills [see Warnings and Precautions (5.1)]. Patients at risk for abuse may include those with prolonged use of BELSOMRA, those with a history of drug abuse, and those who use BELSOMRA in combination with alcohol or other abused drugs.
Drug abuse is the intentional non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than to other activities and obligations), and possible tolerance or physical dependence.
In an abuse liability study conducted in recreational polydrug users (n=36), suvorexant (40, 80 and 150 mg) produced similar effects as zolpidem (15, 30 mg) on subjective ratings of "drug liking" and other measures of subjective drug effects. Because individuals with a history of abuse or addiction to alcohol or other drugs may be at increased risk for abuse and addiction to BELSOMRA, follow such patients carefully.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. In completed clinical trials with BELSOMRA, there was no evidence for physical dependence with the prolonged use of BELSOMRA. There were no reported withdrawal symptoms after discontinuation of BELSOMRA.
10. Overdosage
There is limited premarketing clinical experience with an overdosage of BELSOMRA. In clinical pharmacology studies, healthy subjects who were administered morning doses of up to 240 mg of suvorexant showed dose-dependent increases in the frequency and duration of somnolence.
General symptomatic and supportive measures should be used, along with immediate gastric lavage where appropriate. Intravenous fluids should be administered as needed. As in all cases of drug overdose, vital signs should be monitored and general supportive measures employed. The value of dialysis in the treatment of overdosage has not been determined. As suvorexant is highly protein-bound, hemodialysis is not expected to contribute to elimination of suvorexant.
As with the management of all overdosage, the possibility of multiple drug ingestion should be considered. Consider contacting a poison control center for up-to-date information on the management of hypnotic drug product overdosage.
11. Belsomra Description
BELSOMRA tablets contain suvorexant, an orexin receptor antagonist.
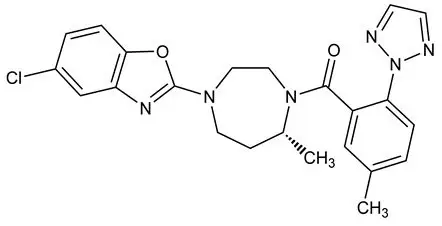
Suvorexant is described chemically as:
[(7R)-4-(5-chloro-2-benzoxazolyl) hexahydro-7-methyl-1H-1,4-diazepin-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone
Its empirical formula is C23H23ClN6O2 and the molecular weight is 450.92. Its structural formula is:
Suvorexant is a white to off-white powder that is insoluble in water.
Each film coated tablet contains 5 mg, 10 mg, 15 mg, or 20 mg of suvorexant and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and polyvinylpyrrolidone/vinyl acetate copolymer (copovidone).
In addition, the film coating contains the following inactive ingredients: hypromellose, lactose monohydrate, titanium dioxide, and triacetin. The film coating for the 5 mg tablets also contains iron oxide black and iron oxide yellow, and the film coating for the 10 mg tablets also contains FD&C Blue #1/Brilliant Blue FCF Aluminum Lake and iron oxide yellow.
12. Belsomra - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of suvorexant in the treatment of insomnia is presumed to be through antagonism of orexin receptors. The orexin neuropeptide signaling system plays a role in wakefulness. Blocking the binding of wake-promoting neuropeptides orexin A and orexin B to receptors OX1R and OX2R is thought to suppress wake drive.
12.2 Pharmacodynamics
Suvorexant binds to orexin receptors, OX1R and OX2R (Ki= 0.55 and 0.35 nM, respectively).
Antagonism of orexin receptors may also underlie potential adverse effects such as signs of narcolepsy/cataplexy. Genetic mutations in the orexin system in animals result in hereditary narcolepsy; loss of orexin neurons has been reported in humans with narcolepsy.
12.3 Pharmacokinetics
Suvorexant exposure increases in a less than strictly dose-proportional manner over the range of 10-80 mg because of decreased absorption at higher doses. Suvorexant pharmacokinetics are similar in healthy subjects and patients with insomnia.
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Suvorexant administered to dogs at oral doses of 5 and 30 mg/kg/day for 4 to 7 days resulted in behaviors characteristic of cataplexy (e.g., transient limb buckling, prone posture) when presented with food enrichment, a stimulus demonstrated to induce cataplexy in dogs with hereditary narcolepsy.
In the 2-year carcinogenicity study in rats, an increased incidence of retinal atrophy was observed at all doses. Plasma AUCs at the lowest dose tested were approximately 7 times that in humans at the MRHD.
In subsequent studies of suvorexant in albino and pigmented rats, retinal atrophy was delayed in onset and, after approximately one year of dosing, was of lower incidence and severity in pigmented rats.
14. Clinical Studies
14.1 Controlled Clinical Studies
BELSOMRA was evaluated in three clinical trials in patients with insomnia characterized by difficulties with sleep onset and sleep maintenance.
Two similarly designed, 3-month, randomized, double-blind, placebo-controlled, parallel-group studies were conducted (Study 1 and Study 2). In both studies, non-elderly (age 18-64) and elderly (age ≥ 65) patients were randomized separately. For the studies together, non-elderly adults (mean age 46 years; 465 females, 275 males) were treated with BELSOMRA 20 mg (n=291) or placebo (n=449). Elderly patients (mean age 71 years, 346 females, 174 males) were treated with BELSOMRA 15 mg (n=202) or placebo (n=318).
In Study 1 and Study 2, BELSOMRA 15 mg or 20 mg was superior to placebo for sleep latency as assessed both objectively by polysomnography (Table 3) and subjectively by patient-estimated sleep latency (Table 4). BELSOMRA 15 mg or 20 mg was also superior to placebo for sleep maintenance, as assessed both objectively by polysomnography (Table 5) and subjectively by patient-estimated total sleep time (Table 6). The effects of BELSOMRA at night 1 (objective) and week 1 (subjective) were generally consistent with later time points. The efficacy of BELSOMRA was similar between women and men and, based on limited data, between Caucasians and non-Caucasians. Twenty seven percent of patients treated with BELSOMRA 15 mg or 20 mg in Study 1 and Study 2 were non-Caucasians. The majority (69%) of the non-Caucasian patients was Asian.
| Mean Baseline and Change from Baseline†
After 1 and 3 Months (minutes) | Difference† Between BELSOMRA and Placebo (minutes) |
||
|---|---|---|---|
| † Change from baseline and treatment differences based upon estimated means. | |||
| ‡ 15 mg in elderly and 20 mg in non-elderly patients | |||
| * p<0.05; **p<0.01; ***p<0.001 | |||
| Study 1 | |||
| Placebo (n=290) | BELSOMRA 15-20 mg‡
(n=193) | ||
| Baseline | 66 | 69 | |
| Change from Baseline | |||
| Month 1 | - 23 | - 34 | - 10*** |
| Month 3 | - 27 | - 35 | - 8** |
| Study 2 | |||
| Placebo (n=286) | BELSOMRA 15-20 mg‡
(n=145) | ||
| Baseline | 69 | 65 | |
| Change from Baseline | |||
| Month 1 | - 25 | - 33 | - 8* |
| Month 3 | - 29 | - 29 | 0 |
| Mean Baseline and Change from Baseline†
After 1 and 3 Months (minutes) | Difference† Between BELSOMRA and Placebo (minutes) |
||
|---|---|---|---|
| † Change from baseline and treatment differences based upon estimated means. | |||
| ‡ 15 mg in elderly and 20 mg in non-elderly patients | |||
| * p<0.05; **p<0.01; ***p<0.001 | |||
| Study 1 | |||
| Placebo (n=382) | BELSOMRA 15-20 mg‡
(n=251) | ||
| Baseline | 67 | 64 | |
| Change from Baseline | |||
| Month 1 | - 12 | - 17 | - 5 |
| Month 3 | - 17 | - 23 | - 5* |
| Study 2 | |||
| Placebo (n=369) | BELSOMRA 15-20 mg‡
(n=231) | ||
| Baseline | 83 | 86 | |
| Change from Baseline | |||
| Month 1 | - 14 | - 21 | - 7* |
| Month 3 | - 21 | - 28 | - 8* |
| Mean Baseline and Change from Baseline†
After 1 and 3 Months (minutes) | Difference† Between BELSOMRA and Placebo (minutes) |
||
|---|---|---|---|
| † Change from baseline and treatment differences based upon estimated means. | |||
| ‡ 15 mg in elderly and 20 mg in non-elderly patients | |||
| * p<0.05; **p<0.01; ***p<0.001 | |||
| Study 1 | |||
| Placebo (n=290) | BELSOMRA 15-20 mg‡
(n=193) | ||
| Baseline | 115 | 120 | |
| Change from Baseline | |||
| Month 1 | - 19 | - 45 | - 26*** |
| Month 3 | - 25 | - 42 | - 17*** |
| Study 2 | |||
| Placebo (n=286) | BELSOMRA 15-20 mg‡
(n=145) | ||
| Baseline | 118 | 119 | |
| Change from Baseline | |||
| Month 1 | - 23 | - 47 | - 24*** |
| Month 3 | - 25 | - 56 | - 31*** |
| Mean Baseline and Change from Baseline†
After 1 and 3 Months (minutes) | Difference† Between BELSOMRA and Placebo (minutes) |
||
|---|---|---|---|
| † Change from baseline and treatment differences based upon estimated means. | |||
| ‡ 15 mg in elderly and 20 mg in non-elderly patients | |||
| * p<0.05; **p<0.01; ***p<0.001 | |||
| Study 1 | |||
| Placebo (n=382) | BELSOMRA 15-20 mg‡
(n=251) | ||
| Baseline | 315 | 322 | |
| Change from Baseline | |||
| Month 1 | 23 | 39 | 16*** |
| Month 3 | 41 | 51 | 11* |
| Study 2 | |||
| Placebo (n=369) | BELSOMRA 15-20 mg‡
(n=231) | ||
| Baseline | 307 | 299 | |
| Change from Baseline | |||
| Month 1 | 22 | 43 | 21*** |
| Month 3 | 38 | 60 | 22*** |
In the 1-month crossover study (Study 3), non-elderly adults (age 18-64 years, mean age 44 years) were treated with placebo (n=249) and BELSOMRA at a dose of 10 mg (n=62), 20 mg (n=61), or up to 80 mg. BELSOMRA 10 mg and 20 mg were superior to placebo for sleep latency and sleep maintenance, as assessed objectively by polysomnography.
BELSOMRA was also evaluated at doses of 30 mg and 40 mg in the 3-month placebo-controlled trials (Study 1 and Study 2). The higher doses were found to have similar efficacy to lower doses, but significantly more adverse reactions were reported at the higher doses.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 2/2023 |
| MEDICATION GUIDE BELSOMRA® (bell-SOM-rah) suvorexant Tablets C-IV |
|
| What is the most important information I should know about BELSOMRA? BELSOMRA may cause serious side effects including:
See "What are the possible side effects of BELSOMRA?" for more information about side effects. |
|
What is BELSOMRA?
BELSOMRA is a federally controlled substance (C-IV) because it can be abused or cause dependence. Keep BELSOMRA in a safe place to prevent misuse and abuse. Selling or giving away BELSOMRA may harm others and is against the law. Tell your doctor if you have ever abused or have been dependent on alcohol, prescription medicines or street drugs. |
|
| Who should not take BELSOMRA? | |
| Do not take BELSOMRA if you fall asleep often at unexpected times (narcolepsy). | |
| What should I tell my doctor before taking BELSOMRA? | |
Before taking BELSOMRA, tell your doctor about all of your medical conditions, including if you:
|
|
| Tell your doctor about all the medicines you take, including prescription or over-the-counter medicines, vitamins, or herbal supplements. Medicines can interact with each other, sometimes causing serious side effects. Do not take BELSOMRA with other medicines that can make you sleepy unless your doctor tells you to. | |
| Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine. | |
How should I take BELSOMRA?
|
|
What should I avoid while taking BELSOMRA?
|
|
| What are the possible side effects of BELSOMRA? See "What is the most important information I should know about BELSOMRA?" BELSOMRA may cause serious side effects including: |
|
|
|
| The most common side effects of BELSOMRA include drowsiness the next day after you take BELSOMRA. The following additional side effects have been reported with BELSOMRA: abnormal dreams. |
|
|
These are not all the possible side effects of BELSOMRA. For more information, ask your doctor or pharmacist. |
|
| Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
How should I store BELSOMRA?
|
|
| General information about the safe and effective use of BELSOMRA. | |
| Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BELSOMRA for a condition for which it was not prescribed. Do not give BELSOMRA to other people, even if they have the same symptoms that you have. It may harm them. | |
| This Medication Guide summarizes the most important information about BELSOMRA. You can ask your pharmacist or doctor for information about BELSOMRA that is written for health professionals. | |
| For more information, go to www.BELSOMRA.com or call 1-800-622-4477. | |
| What are the ingredients in BELSOMRA? | |
| Active ingredient: Suvorexant | |
| Inactive ingredients: Croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and polyvinylpyrrolidone/vinyl acetate copolymer (copovidone). The film coating contains: hypromellose, lactose monohydrate, titanium dioxide, and triacetin. The film coating for the 5 mg tablets also contains iron oxide black and iron oxide yellow, and the film coating for the 10 mg tablets also contains FD&C Blue #1/Brilliant Blue FCF Aluminum Lake and iron oxide yellow. | |
| Distributed by: Merck Sharp & Dohme LLC Rahway, NJ 07065, USA |
|
| For patent information: www.msd.com/research/patent | |
| Copyright © 2014-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved. |
|
| usmg-mk4305-t-2302r006 | |
| BELSOMRA
suvorexant tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| BELSOMRA
suvorexant tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| BELSOMRA
suvorexant tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| BELSOMRA
suvorexant tablet, film coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |