Drug Detail:Benzamycin (Benzoyl peroxide and erythromycin topical [ ben-zoe-ill-per-ox-ide-and-er-ith-roe-mye-sin ])
Drug Class: Topical acne agents
Topical Gel: erythromycin (3%), benzoyl peroxide (5%)
For Topical Use Only – Not for Ophthalmic Use
Reconstitute Before Dispensing
Benzamycin Gel Description
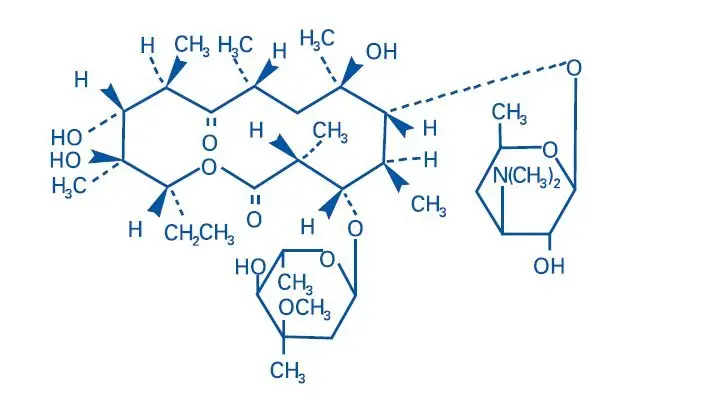
Benzamycin® Topical Gel contains erythromycin [(3R*,4S*,5S*,6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)-oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione]. Erythromycin is a macrolide antibiotic produced from a strain of Saccharopolyspora erythraea (formerly Streptomyces erythreus). It is a base and readily forms salts with acids.
Chemically erythromycin is C37H67NO13. It has the following structural formula:
Erythromycin has the molecular weight of 733.94. It is a white crystalline powder and has a solubility of approximately 1 mg/mL in water and is soluble in alcohol at 25°C.
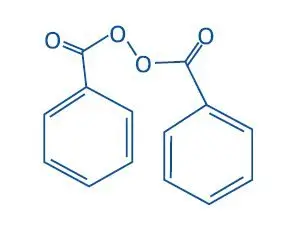
Benzamycin Topical Gel also contains benzoyl peroxide for topical use. Benzoyl peroxide is an antibacterial and keratolytic agent.
Chemically benzoyl peroxide is C14H10O4. It has the following structural formula:
Benzoyl peroxide has the molecular weight of 242.23. It is a white granular powder and is sparingly soluble in water and alcohol and soluble in acetone, chloroform and ether.
Each gram of Benzamycin Topical Gel contains, as dispensed, 30 mg (3%) of erythromycin and 50 mg (5%) of benzoyl peroxide in a base of purified water USP, Carbomer Homopolymer Type C, alcohol 20%, sodium hydroxide NF, docusate sodium and fragrance.
Related/similar drugs
doxycycline, clindamycin topical, erythromycin topical, tetracycline, TazoracBenzamycin Gel - Clinical Pharmacology
The exact mechanism by which erythromycin reduces lesions of acne vulgaris is not fully known; however, the effect appears to be due in part to the antibacterial activity of the drug.
Benzoyl peroxide has a keratolytic and desquamative effect which may also contribute to its efficacy. Benzoyl peroxide has been shown to be absorbed by the skin where it is converted to benzoic acid.
MICROBIOLOGY
Erythromycin acts by inhibition of protein synthesis in susceptible organisms by reversibly binding to 50 S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. Antagonism has been demonstrated in vitro between erythromycin, lincomycin, chloramphenicol and clindamycin.
Benzoyl peroxide is an antibacterial agent which has been shown to be effective against Propionibacterium acnes, an anaerobe found in sebaceous follicles and comedones. The antibacterial action of benzoyl peroxide is believed to be due to the release of active oxygen.
Indications and Usage for Benzamycin Gel
Benzamycin Topical Gel is indicated for the topical treatment of acne vulgaris.
Contraindications
Benzamycin Topical Gel is contraindicated in those individuals who have shown hypersensitivity to any of its components.
Warnings
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including erythromycin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of “antibiotic-associated colitis”.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation and treatment with an antibacterial drug clinically effective against C. difficile colitis.
Precautions
General:
For topical use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating or abrasive agents. If severe irritation develops, discontinue use and institute appropriate therapy.
The use of antibiotic agents may be associated with the overgrowth of nonsusceptible organisms including fungi. If this occurs, discontinue use and take appropriate measures.
Avoid contact with eyes and all mucous membranes.
Information for Patients:
Patients using Benzamycin Topical Gel should receive the following information and instructions:
- 1.
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes, nose, mouth, and all mucous membranes.
- 2.
- This medication should not be used for any disorder other than that for which it was prescribed.
- 3.
- Patients should not use any other topical acne preparation unless otherwise directed by their physician.
- 4.
- Patients should report to their physician any signs of local adverse reactions.
- 5.
- Benzamycin Topical Gel may bleach hair or colored fabric.
- 6.
- Keep product refrigerated and discard after 3 months.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Data from a study using mice known to be highly susceptible to cancer suggests that benzoyl peroxide acts as a tumor promoter. The clinical significance of this is unknown.
No animal studies have been performed to evaluate the carcinogenic and mutagenic potential or effects on fertility of topical erythromycin. However, long-term (2-year) oral studies in rats with erythromycin ethylsuccinate and erythromycin base did not provide evidence of tumorigenicity. There was no apparent effect on male or female fertility in rats fed erythromycin (base) at levels up to 0.25% of diet.
Pregnancy:
Teratogenic Effects:
Animal reproduction studies have not been conducted with Benzamycin Topical Gel or benzoyl peroxide.
There was no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base (up to 0.25% diet) prior to and during mating, during gestation and through weaning of two successive litters.
There are no well-controlled trials in pregnant women with Benzamycin Topical Gel. It also is not known whether Benzamycin Topical Gel can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Benzamycin Topical Gel should be given to a pregnant woman only if clearly needed.
Nursing Women:
It is not known whether Benzamycin Topical Gel is excreted in human milk after topical application. However, erythromycin is excreted in human milk following oral and parenteral erythromycin administration. Therefore, caution should be exercised when erythromycin is administered to a nursing woman.
Adverse Reactions/Side Effects
In controlled clinical trials, the incidence of adverse reactions associated with the use of Benzamycin Topical Gel was approximately 3%. These were dryness and urticarial reaction.
The following additional local adverse reactions have been reported occasionally: irritation of the skin including peeling, itching, burning sensation, erythema, inflammation of the face, eyes and nose, and irritation of the eyes. Skin discoloration, oiliness and tenderness of the skin have also been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Benzamycin Gel Dosage and Administration
Benzamycin Topical Gel should be applied twice daily, morning and evening, or as directed by a physician, to affected areas after the skin is thoroughly washed, rinsed with warm water and gently patted dry.
How is Benzamycin Gel supplied
|
Size |
Benzoyl Peroxide Gel |
Active Erythromycin Powder (In Plastic Vial) |
70% Ethyl Alcohol To Be Added |
|
|
(Net Weight) |
NDC |
|||
|
46.6 grams |
0187-5205-46 |
40 grams |
1.6 grams |
6 mL |
|
(as dispensed) |
Prior to dispensing, tap vial until all powder flows freely. Add indicated amount of room temperature 70% ethyl alcohol to vial (to the mark) and immediately shake to completely dissolve erythromycin. Add this solution to gel and stir until homogeneous in appearance (1 to 1½ minutes). Benzamycin Topical Gel should then be stored under refrigeration. Do not freeze. Place a 3-month expiration date on the label.
NOTE: Prior to reconstitution, store at room temperature between 15° to 30°C (59° to 86°F).
After reconstitution, store under refrigeration between 2° to 8°C (36° to 46°F).
Do not freeze. Keep tightly closed. Keep out of reach of children.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
BENZAMYCIN is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
|
PLEASE READ COMPLETE COMPOUNDING DIRECTIONS |
|
NOTE: TAP VIAL UNTIL ALL POWDER FLOWS FREELY. ADD ROOM TEMPERATURE 70% ETHYL ALCOHOL TO VIAL (TO THE MARK) AND IMMEDIATELY SHAKE/DISSOLVE COMPLETELY. |
Rev. 11/2020
9560802
| BENZAMYCIN
erythromycin and benzoyl peroxide kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bausch Health US, LLC (831922468) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch Health Companies Inc. | 245141858 | MANUFACTURE(0187-5205) , ANALYSIS(0187-5205) , LABEL(0187-5205) , PACK(0187-5205) | |