BenzePrO Creamy Wash Description
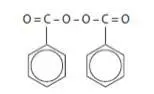
BenzePrOTM Creamy Wash is intended for topical administration and contains Benzoyl Peroxide for use in the treatment of acne vulgaris. Benzoyl Peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl Peroxide (C14H10O4) is represented by the following chemical structure:
Each gram of BenzePrOTM Creamy Wash contains 70 mg of Benzoyl Peroxide as microsphere particles (via Curoxyl® 42) in an emulsion based formulation consisting of: aloe, carbomer 940, cetyl alcohol, disodium oleamido MEA-sulfosuccinate, disodium EDTA, glyceryl stearate/PEG-100 stearate, glycerin, green tea, laureth-12, magnesium aluminum silicate, propylene glycol, purified water, sodium coco-sulfate, sodium lauroamphoacetate, xanthan gum.