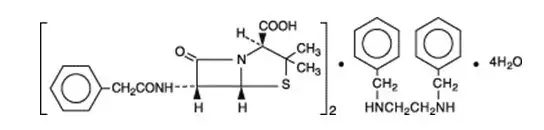
Drug Class: Natural penicillins
WARNING: NOT FOR INTRAVENOUS USE. DO NOT INJECT INTRAVENOUSLY OR ADMIX WITH OTHER INTRAVENOUS SOLUTIONS. THERE HAVE BEEN REPORTS OF INADVERTENT INTRAVENOUS ADMINISTRATION OF PENICILLIN G BENZATHINE WHICH HAS BEEN ASSOCIATED WITH CARDIORESPIRATORY ARREST AND DEATH. Prior to administration of this drug, carefully read the WARNINGS, ADVERSE REACTIONS, and DOSAGE AND ADMINISTRATION sections of the labeling.
Bicillin C-R - Clinical Pharmacology
Microbiology
Indications and Usage for Bicillin C-R
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Bicillin C-R and other antibacterial drugs, Bicillin C-R should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
This drug is indicated in the treatment of moderately severe infections due to penicillin-G-susceptible microorganisms that are susceptible to serum levels common to this particular dosage form. Therapy should be guided by bacteriological studies (including susceptibility testing) and by clinical response.
Bicillin C-R is indicated in the treatment of the following in adults and pediatric patients:
Moderately severe to severe infections of the upper-respiratory tract, scarlet fever, erysipelas, and skin and soft-tissue infections due to susceptible streptococci.
NOTE: Streptococci in Groups A, C, G, H, L, and M are very sensitive to penicillin G. Other groups, including Group D (enterococci), are resistant. Penicillin G sodium or potassium is recommended for streptococcal infections with bacteremia.
Moderately severe pneumonia and otitis media due to susceptible Streptococcus pneumoniae.
NOTE: Severe pneumonia, empyema, bacteremia, pericarditis, meningitis, peritonitis, and arthritis of pneumococcal etiology are better treated with penicillin G sodium or potassium during the acute stage.
When high, sustained serum levels are required, penicillin G sodium or potassium, either IM or IV, should be used. This drug should not be used in the treatment of venereal diseases, including syphilis, gonorrhea, yaws, bejel, and pinta.
Warnings
| WARNING: NOT FOR INTRAVENOUS USE. DO NOT INJECT INTRAVENOUSLY OR ADMIX WITH OTHER INTRAVENOUS SOLUTIONS. THERE HAVE BEEN REPORTS OF INADVERTENT INTRAVENOUS ADMINISTRATION OF PENICILLIN G BENZATHINE WHICH HAS BEEN ASSOCIATED WITH CARDIORESPIRATORY ARREST AND DEATH. Prior to administration of this drug, carefully read the WARNINGS, ADVERSE REACTIONS, and DOSAGE AND ADMINISTRATION sections of the labeling. |
The combination of penicillin G benzathine and penicillin G procaine should only be prescribed for the indications listed in this insert.
PRINCIPAL DISPLAY PANEL - 2 mL Syringe Label - 601
NDC 60793-601-02
BICILLIN® C-R
(600,000 units penicillin G benzathine and
600,000 units penicillin G procaine injectable
suspension)
1,200,000 units per 2 mL
Not for the Treatment of Syphilis
FOR DEEP INTRAMUSCULAR INJECTION ONLY
WARNING: FATAL IF GIVEN BY OTHER ROUTES
Distributed by
Pfizer Inc
New York, NY 10017

PRINCIPAL DISPLAY PANEL - 2 mL Syringe Package - 601
NDC 60793-601-10
Contains 10 of NDC 60793-601-02
Ten Syringes (2 mL size)
Bicillin® C-R
(penicillin G benzathine and penicillin G procaine
injectable suspension)
1,200,000 units per 2 mL
FOR PEDIATRIC USE
FOR DEEP INTRAMUSCULAR INJECTION ONLY
WARNING: FATAL IF GIVEN BY OTHER ROUTES
BEFORE INJECTING, SEE PACKAGE INSERT FOR ADMINISTRATION INSTRUCTIONS.
NOT FOR THE TREATMENT OF SYPHILIS
Pfizer Injectables
Rx only

PRINCIPAL DISPLAY PANEL - 2 mL Syringe Label - 600
NDC 60793-600-02
BICILLIN® C-R
(penicillin G benzathine and penicillin G
procaine injectable suspension in equal
portions)
1,200,000 units per 2 mL
Not for the Treatment of Syphilis
FOR DEEP INTRAMUSCULAR INJECTION ONLY
WARNING: FATAL IF GIVEN BY OTHER ROUTES
Distributed by
Pfizer Inc
New York, NY 10017

PRINCIPAL DISPLAY PANEL - 2 mL Syringe Package - 600
NDC 60793-600-10
Contains 10 of NDC 60793-600-02
Ten Syringes (2 mL size)
Bicillin® C-R
(penicillin G benzathine and penicillin G procaine
injectable suspension)
1,200,000 units per 2 mL
FOR DEEP INTRAMUSCULAR INJECTION ONLY
WARNING: FATAL IF GIVEN BY OTHER ROUTES
BEFORE INJECTING, SEE PACKAGE INSERT FOR ADMINISTRATION INSTRUCTIONS.
NOT FOR THE TREATMENT OF SYPHILIS
Pfizer Injectables
Rx only

| BICILLIN CR
penicillin g benzathine and penicillin g procaine injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| BICILLIN CR
penicillin g benzathine and penicillin g procaine injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| King Pharmaceuticals LLC | 962691478 | ANALYSIS(60793-600, 60793-601) , MANUFACTURE(60793-600, 60793-601) , PACK(60793-600, 60793-601) , LABEL(60793-600, 60793-601) | |