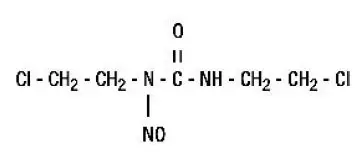
Drug Detail:Bicnu (injection/implant) (Carmustine (injection/implant) [ kar-mus-teen ])
Drug Class: Alkylating agents
Highlights of Prescribing Information
BiCNU (carmustine) for injection, for intravenous use
Initial U.S. Approval: 1977
WARNING: MYELOSUPPRESSION and PULMONARY TOXICITY
See full prescribing information for complete boxed warning
• Suppression of marrow function, notably thrombocytopenia and leukopenia, is the most common and severe of the toxic effects of BiCNU. Monitor blood counts. (5, 6).
• Pulmonary toxicity from BiCNU appears to be dose related. Patients receiving greater than 1400 mg/m2 cumulative dose are at significantly higher risk than those receiving less (5, 6).
Indications and Usage for BiCNU
BiCNU is a nitrosourea indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in the following: (1)
- Brain tumors glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors (1)
- Multiple myeloma-in combination with prednisone (1)
- Relapsed or refractory Hodgkin's lymphoma in combination with other approved drugs (1)
- Relapsed or refractory Non-Hodgkin's lymphomas in combination with other approved drugs (1)
BiCNU Dosage and Administration
- Recommended Dosage: As a single agent, 150 to 200 mg/m2BiCNU intravenously every 6 weeks as a single dose or divided into daily injections such as 75 to 100 mg/m2on 2 successive days. Adjust dose for combination therapy or in patients with reduced bone marrow reserve (2.1)
- Administer reconstituted solution only as a slow intravenous infusion over at least 2 hours. (2.2)
Dosage Forms and Strengths
For injection: 100 mg of carmustine lyophilized powder in a single-dose vial for reconstitution and a vial containing 3mL sterile diluent (Dehydrated Alcohol Injection, USP) (3)
Contraindications
- Hypersensitivity (4)
Warnings and Precautions
- Administration Reactions: Extravasation may occur; monitor infusion site closely during administration (5.3)
- Carcinogenicity: Potentially carcinogenic to humans. Monitor patient periodically for such signs and apprise the patient of the symptoms for which they need to seek medical help. (5.4)
- Ocular Toxicity: Has occurred when administered via unapproved intraarterial intracarotid route. (5.5)
- Embryo-Fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to avoid pregnancy. (5.6)
Adverse Reactions/Side Effects
Most common adverse reactions (>1%) are nausea, vomiting, renal toxicity, pneumonitis, pulmonary toxicity, myelosuppression (6)
To report SUSPECTED ADVERSE REACTIONS, contact Avet Pharmaceuticals Inc. at 1-866-901-DRUG (3784) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Cimetidine: Increased myelosuppression with concomitant use. (7.1)
- Phenobarbital: Induces carmustine metabolism, reducing exposure. May lead to reduced efficacy. (7.1)
- Phenytoin: BiCNU may reduce the efficacy of phenytoin. (7.2)
Use In Specific Populations
- Lactation: Advise lactating females not to breastfeed (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2021
Related/similar drugs
methotrexate, Keytruda, Avastin, rituximab, pembrolizumab, cisplatin, cyclophosphamideFull Prescribing Information
WARNING: MYELOSUPPRESSION and PULMONARY TOXICITY
BiCNU causes suppression of marrow function (including thrombocytopenia and leukopenia), which may contribute to bleeding and overwhelming infections. [see Warnings and Precautions (5.1) and Adverse Reactions (6)]. Monitor blood counts weekly for at least 6 weeks after each dose. Adjust dosage based on nadir blood counts from the prior dose [see Dosage and Administration (2.1)]. Do not administer a repeat course of BiCNU until blood counts recover.
Pulmonary Toxicity
BiCNU causes dose-related pulmonary toxicity. Patients receiving greater than 1400 mg/m2cumulative dose are at significantly higher risk than those receiving less. Delayed pulmonary toxicity can occur years after treatment, and can result in death, particularly in patients treated in childhood [see Adverse Reactions (6) and Use in Specific Populations (8.4)].
1. Indications and Usage for BiCNU
- Brain tumors glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors.
- Multiple myeloma in combination with prednisone.
- Relapsed or refractory Hodgkin's lymphoma in combination with other approved drugs.
- Relapsed or refractory Non-Hodgkin's lymphomas in combination with other approved drugs.
2. BiCNU Dosage and Administration
2.1 Dosage
Adjust doses subsequent to the initial dose according to the hematologic response of the patient to the preceding dose. The following schedule is suggested as a guide to dosage adjustment:
| Nadir After Prior Dose | Percentage of Prior Dose to be Given |
|
| Leukocytes/mm3
| Platelets/mm3
| |
| >4000 | >100,000 | 100% |
| 3000-3999 | 75,000-99,999 | 100% |
| 2000-2999 | 25,000-74,999 | 70% |
| <2000 | <25,000 | 50% |
Evaluate renal function prior to administration and periodically during treatment. For patients with compromised renal function, monitor for toxicity more frequently. Discontinue BiCNU if the creatinine clearance is less than 10 mL/min. Do not administer BiCNU to patients with compromised renal function. Monitor transaminases and bilirubin periodically during treatment. [see Adverse Reactions (6)].
2.2 Preparation and Administration of Intravenous Solution
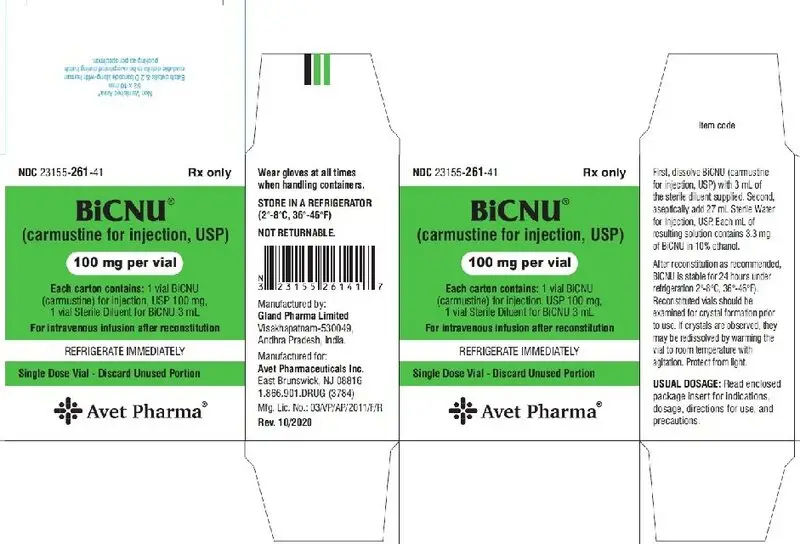
- Dissolve BiCNU with 3 mL of the supplied sterile diluent (Dehydrated Alcohol Injection, USP).
- Aseptically add 27 mL Sterile Water for Injection, USP.
- Each mL of resulting solution contains 3.3 mg of BiCNU in 10% ethanol. Such solutions should be protected from light.
- The reconstituted solution is a clear, colorless to yellowish solution.
- Once reconstituted, the solution must be further diluted with Sodium Chloride Injection, USP or 5% Dextrose Injection, USP.
- Examine reconstituted vials for crystal formation prior to use. If crystals are observed, they may be re-dissolved by warming the vial to room temperature with agitation.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- After reconstitution as recommended, BiCNU is stable for 24 hours under refrigeration (2°-8°C, 36°-46°F) in glass container. Examine reconstituted vials for crystal formation prior to use. If crystals are observed, they may be redissolved by warming the vial to room temperature with agitation.
- Vials reconstituted as directed and further diluted with 500 mL Sodium Chloride Injection, USP or 5% Dextrose Injection, USP, in glass or polypropylene containers to a concentration of 0.2 mg/mL, should be stored at room temperature, protected from light and utilized within 8 hours. These solutions are also stable 24 hours under refrigeration (2°-8°C, 36°-46°F) and an additional 6 hours at room temperature protected from light.
- Administer reconstituted solution by slow intravenous infusion over at least two hours. Administration of BiCNU over a period of less than two hours can lead to pain and burning at the site of injection. Monitor the injected area during the administration. The rate of administration of the intravenous infusion should not be more than 1.66 mg/m2/min.
- See Section 16.2 for important instructions on the storage and handling of the injection. BiCNU is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
- The lyophilized dosage formulation contains no preservatives and is not intended for use as a multiple dose via.
5. Warnings and Precautions
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Pulmonary toxicity [see Warnings and Precautions (5.2)]
- Administration Reactions [see Warnings and Precautions (5.3)]
- Carcinogenicity [see Warnings and Precautions (5.4)]
- Ocular Toxicity [see Warnings and Precautions (5.5)]
Cardiac Disorders
Tachycardia and chest pain.
Eye Disorders
Conjunctival edema, conjunctival hemorrhage, blurred vision and loss of depth perception
Gastrointestinal Toxicity
Nausea, vomiting, anorexia, and diarrhea
Hepatotoxicity
Increased transaminase, increased alkaline phosphatase, increased bilirubin levels
Infections and Infestations
Opportunistic infection (including with fatal outcome).
Neoplasms Benign, Malignant and Unspecified (including cysts and polyps)
Acute leukemia, bone marrow dysplasias.
Nephrotoxicity
Progressive azotemia, decrease in kidney size, renal failure
Nervous System Disorders
Headaches, encephalopathy, and seizures
Pulmonary Toxicity
Pneumonitis, interstitial lung disease
Reproductive System and Breast Disorders
Gynecomastia
Skin and Subcutaneous Tissue Disorders
Burning sensation, hyperpigmentation, swelling, pain, erythema, skin necrosis, alopecia, allergic reaction
Vascular Disorders
Veno-occlusive disease.
7. Drug Interactions
8. Use In Specific Populations
8.1 Pregnancy
BiCNU (carmustine for injection) can cause fetal harm when administered to a pregnant woman based on the mechanism of action [see Clinical Pharmacology (12.1)] and findings in animals [see Data]. Limited available data with BiCNU use in pregnant women are insufficient to inform a drug-associated risk of major birth defects and miscarriage. Carmustine was embryotoxic in rats and rabbits and teratogenic in rats (thoracoabdominal closure, neural tube, and eye defects and malformations of the skeletal system of the fetus) when given in doses lower than the maximum cumulative human dose based on body surface area. Consider the benefits and risks of BiCNU for the mother and possible risks to the fetus when prescribing BiCNU to a pregnant woman.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Intraperitoneal (IP) administration of carmustine to pregnant rats 14 days prior to mating and during the period of organogenesis at cumulative doses ≥ 26 mg/kg (158 mg/ m2), approximately 0.1 times the maximum cumulative human dose of 1400 mg/m2, resulted in pre-implantation loss, increased resorptions (including completely resorbed litters), and reduced the number of live births in the presence of maternal toxicity.
Carmustine administered IP to pregnant rats during the period of organogenesis at cumulative doses ≥ 4 mg/kg (24 mg/m2), approximately 0.02 times the maximum cumulative human dose based on a mg/m2 basis, resulted in reduced fetal weight and various malformations, which included thoracoabdominal closure defects, neural tube defects, and eye defects, including microphthalmia/anophthalmia, and skeletal anomalies in the skull, sternebra, vertebrae and ribs, and reduced skeletal ossification) in the presence of maternal toxicity. Embryo-fetal death was observed at cumulative doses ≥ 8 mg/kg (48 mg/m2), approximately 0.03 times the maximum cumulative human dose on a mg/ m2 basis. Intravenous (IV) administration of carmustine to rats at a cumulative dose of 50 mg/kg (300 mg/ m2), approximately 0.2 times the maximum cumulative human dose on a mg/m2 basis, during the last quarter of pregnancy resulted in the death of offspring within 4 months. Carmustine administered IV to rabbits during the period of organogenesis resulted in spontaneous abortions in mothers and growth defects in the fetus, mainly at cumulative doses ≥ 13 mg/kg (156 mg/ m2), approximately 0.1 times the maximum cumulative human dose on a mg/ m2 basis.
8.2 Lactation
There is no information regarding the presence of carmustine in human milk, the effects on the breastfed infant, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse events (e.g., carcinogenicity and myelosuppression) in nursing infants, nursing should be discontinued while taking BiCNU.
8.3 Females and Males of Reproductive Potential
Advise female patients to avoid pregnancy during treatment with BiCNU because of the risk of fetal harm [see Use in Specific Populations (8.1)].
Advise female patients of reproductive potential to use highly effective contraception during and for up to six months after completion of treatment.
Advise males with female sexual partners of reproductive potential to use effective contraception during BiCNU treatment and for at least three months after the final dose of BiCNU [see Nonclinical Toxicology (13.1)].
Infertility
Based on nonclinical findings, male fertility may be compromised by treatment with BiCNU [see Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
BiCNU and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored.
12. BiCNU - Clinical Pharmacology
12.3 Pharmacokinetics
Carmustine crosses the blood-brain barrier. Levels of radioactivity in the CSF are greater than or equal to 50% of those measured concurrently in plasma.
Elimination
Following a short intravenous infusion, the reported elimination half-life ranges from 15 minutes to 75 minutes.
Metabolism
Carmustine may be inactivated through denitrosation reactions catalyzed by both cytosolic and microsomal enzymes, including NADPH and glutathione-S-transferase.
Excretion
Approximately 60% to 70% of a total dose is excreted in the urine within 96 hours. Approximately 10% is eliminated as respiratory CO2.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carmustine was mutagenic and clastogenic in multiple in vitro and in vivo genetic toxicology studies.
Male rats treated with carmustine at cumulative doses ≥ 36 mg/kg (216 mg/ m2), approximately 0.15 times the maximum cumulative human dose on a mg/ m2 basis, showed decreases in reproductive potential when mated with untreated female rats (e.g., decreased implantations, increased resorption rate, and a decrease in viable fetuses).
16. How is BiCNU supplied
16.2 Storage and Handling
Store product and diluent in a refrigerator (2°-8°C, 36°-46°F).
Stability
Store the unopened vial of the dry drug in a refrigerator (2°-8°C, 36°-46°F). Store the diluent vials in a refrigerator (2°-8°C, 36°-46°F). The recommended storage of unopened BiCNU vials provides a stable product for up to 3 years.
Compatibility/ Incompatibility with Containers
The intravenous solution is unstable in polyvinyl chloride container. DO NOT USE PVC Containers.
Administer BiCNU solution from the glass bottles or polypropylene container only. Ensure the polypropylene containers used are PVC free and DEHP free.
Important Note
BiCNU has a low melting point (30.5°-32.0°C or 86.9°-89.6°F). Exposure of the drug to this temperature or above will cause the drug to liquefy and appear as an oil film on the vials. This is a sign of decomposition and vials should be discarded. If there is a question of adequate refrigeration upon receipt of this product, immediately inspect the vial in each individual carton. Hold the vial to a bright light for inspection. The BiCNU will appear as a very small amount of dry flakes or dry congealed mass. If this is evident, the BiCNU is suitable for use and should be refrigerated immediately.
17. Patient Counseling Information
Myelosuppression [see Warnings and Precautions (5.1)].
A serious and frequent toxicity of BiCNU is delayed myelosuppression and usually occurs 4 to 6 weeks after drug administration. Hence, patients should be advised to get blood counts monitored weekly for at least 6 weeks. The bone marrow toxicity of BiCNU is cumulative.
Pulmonary Toxicity [see Warnings and Precautions (5.2)].
Advise patients to contact a health care professional immediately for any of the following: shortness of breath, particularly during exercise, dry, hacking cough, fast, shallow breathing, gradual unintended weight loss, tiredness, aching joints and muscles, clubbing (widening and rounding) of the tips of the fingers or toes.
Seizures [see Adverse Reactions (6)]
Inform the patient that they may suffer from fits and advise them to get medical attention immediately in such cases.
Pregnancy [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1 and 8.3)]
Advise pregnant women and females of reproductive potential that BiCNU exposure during pregnancy can result in fetal harm. Advise female patients to contact their healthcare provider with a known or suspected pregnancy. Advise women of reproductive potential to avoid becoming pregnant. Advise females of reproductive potential to use effective contraception during treatment.
Lactation [see Use in Specific Populations (8.2)]
Advise the female patient to discontinue nursing while taking BiCNU.
Manufactured by:
Gland Pharma Limited
Visakhapatnam-530049,
Andhra Pradesh, India.
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rx only
Dehydrated Alcohol Injection, USP
3 mL
Sterile Diluent for BiCNU®
(carmustine for injection)

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
BiCNU®
(carmustine for injection)
100 mg per vial
For intravenous infusion after reconstitution
REFRIGERATE IMMEDIATELY
Single Dose Vial - Discard Unused Portion

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rx only
BiCNU®
(carmustine for injection)
100 mg per vial
Each carton contains: 1 vial BiCNU (carmustine) for injection 100 mg, 1 vial Sterile Diluent for BiCNU 3 mL
For intravenous infusion after reconstitution
REFRIGERATE IMMEDIATELY
Single Dose Vial – Discard Unused Portion
| BICNU
carmustine kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. (780779901) |
| Registrant - AVET LIFESCIENCES PRIVATE LIMITED (853181664) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Emcure Pharmaceuticals Limited | 862602830 | ANALYSIS(23155-261, 23155-262, 23155-589) , LABEL(23155-261, 23155-262, 23155-589) , MANUFACTURE(23155-261, 23155-262, 23155-589) , PACK(23155-261, 23155-262, 23155-589) | |