Drug Class: H. pylori eradication agents
Highlights of Prescribing Information
BISMUTH SUBCITRATE POTASSIUM, METRONIDAZOLE and TETRACYCLINE HYDROCHLORIDE CAPSULES, for oral use
Initial U.S. Approval: 2006
WARNING: POTENTIAL FOR CARCINOGENICITY
See full prescribing information for complete boxed warning
Metronidazole has been shown to be carcinogenic in mice and rats. It is unknown whether metronidazole is associated with carcinogenicity in humans (5.1).
Recent Major Changes
| Warnings and Precautions, Drugs Interactions (5.14) | 3/2021 |
| Contraindications (4) | 12/2021 |
Indications and Usage for Bismuth, Metronidazole and Tetracycline Capsules
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules is a combination of metronidazole, a nitroimidazole antimicrobial, tetracycline, - a tetracycline class antimicrobial and bismuth subcitrate potassium, indicated for use, in combination with omeprazole, for the treatment of patients with Helicobacter pylori infection and duodenal ulcer disease (active or history of within the past 5 years) to eradicate H. pylori. (1.1)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules and other antibacterial drugs, Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.2)
Bismuth, Metronidazole and Tetracycline Capsules Dosage and Administration
- Administer three Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules 4 times a day (after meals and at bedtime) for 10 days. (2)
- Administer Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules with omeprazole 20 mg twice daily (after the morning and evening meals). (2)
Dosage Forms and Strengths
Each capsule of Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules contains: (3)
- 140 mg of bismuth subcitrate potassium
- 125 mg metronidazole
- 125 mg of tetracycline hydrochloride
Contraindications
- Concurrent usage of Methoxyflurane. (4.1, 7.1)
- Disulfiram usage within the last two weeks. (4.2, 7.2)
- Alcoholic beverage consumption for at least three days during or after therapy. (4.3, 7.3)
- Patients with Cockayne syndrome. (4.4, 6.3)
- Severe renal impairment. (4.5)
- Women who are pregnant. (4.6, 8.1)
- Known hypersensitivity to product components. (4.7)
Warnings and Precautions
- Fetal Toxicity: Advise pregnant women of the risk throughout pregnancy for retardation of skeletal development seen in animal studies and permanent discoloration of teeth with tetracycline if used during the second or third trimester. (5.2, 8.1)
- Maternal Toxicity: Risk of hepatotoxicity in pregnant women with high doses of intravenous tetracycline also resulting in stillborn or premature birth. (5.3, 8.1)
- Tooth Enamel discoloration and hypoplasia: permanent discoloration may develop with use during tooth development (last half of pregnancy, infancy, and childhood to the age of 8 years). (5.4)
- Central and Peripheral Nervous System Effects: encephalopathy, convulsive seizures, aseptic meningitis and peripheral neuropathy with metronidazole, intracranial hypertension with tetracycline and neurotoxicity with bismuthcontaining products. Monitor patients with CNS conditions closely and discontinue promptly if abnormal neurologic signs develop. (5.5)
- Photosensitivity: avoid exposure to sun and sun lamps. (5.7)
- Blood Dyscrasias: Use with caution in patients with a history of blood dyscrasias. (5.9)
- Hepatic Impairment: Not recommended in patients with severe hepatic impairment. (5.10)
- Cutaneous Reactions: Stevens-Johnson, toxic epidermal necrolysis, DRESS syndrome. Discontinue treatment at the first evidence of a cutaneous reaction. (5.13)
Adverse Reactions/Side Effects
Most frequently reported adverse reactions (≥5%): abnormal feces, diarrhea, nausea, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact the Safety Call Center at 1-833-520-8580 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
- Methoxyflurane: Risk of fatal renal toxicity; do not co-administer. (4.1, 7.1)
- Disulfiram: Psychotic reactions can occur; do not take concurrently or within the last 2 weeks of disulfiram. (4.2, 7.2)
- Alcohol: Abdominal cramps, nausea, vomiting, headaches, and flushing can occur; do not consume during therapy and for at least 3 days afterwards. (4.3, 7.3)
- Oral Contraceptives: Decreased efficacy possibly resulting in pregnancy; use a different or additional form of contraception. (5.14, 7.4)
- Anticoagulants: Potentiation of the anticoagulant effect; Prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored. (5.14, 7.5)
- Lithium: Increased lithium serum concentrations; measure serum lithium and serum creatinine concentrations during therapy. (5.14, 7.6)
- Antacids, Multivitamins or Dairy Products: Decreased absorption of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride; do not take concomitantly. (7.7)
- Busulfan: Increased busulfan serum concentrations; avoid concomitant use, monitor for busulfan toxicity. (7.8)
- CYP inducers and CYP inhibitors: Prolonged or accelerated half-life of metronidazole or concomitant medications; use with caution. (7.9, 7.10)
Use In Specific Populations
- Lactation: A woman should pump and discard human milk for the duration of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride therapy, and for 2 days after therapy ends. (8.2)
- Pediatric Use: Tetracycline may cause permanent discoloration of the teeth. Enamel hypoplasia has also been reported. Do not use in children less than 8 years of age. (5.4, 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2023
Related/similar drugs
omeprazole, amoxicillin, pantoprazole, metronidazole, Nexium, ProtonixFull Prescribing Information
WARNING: POTENTIAL FOR CARCINOGENICITY
Metronidazole has been shown to be carcinogenic in mice and rats. It is unknown whether metronidazole is associated with carcinogenicity in humans [see Warning and Precautions (5.1)].
1. Indications and Usage for Bismuth, Metronidazole and Tetracycline Capsules
1.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules in combination with omeprazole are indicated for the treatment of patients with Helicobacter pylori infection and duodenal ulcer disease (active or history of within the past 5 years) to eradicate H. pylori. The eradication of Helicobacter pylori has been shown to reduce the risk of duodenal ulcer recurrence.
1.2 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules and other antibacterial drugs, Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules should be used to treat only indicated infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. Bismuth, Metronidazole and Tetracycline Capsules Dosage and Administration
Administer three Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules 4 times a day (after meals and at bedtime) for 10 days. One omeprazole 20 mg capsule should be taken twice a day with Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules after the morning and evening meal for 10 days (Table 1).
| Time of dose | Number of capsules of Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules | Number of capsules of omeprazole 20 mg |
|---|---|---|
| After morning meal | 3 | 1 |
| After lunch | 3 | 0 |
| After evening meal | 3 | 1 |
| At bedtime | 3 | 0 |
Instruct patients to swallow the Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules whole with a full glass of water (8 ounces). Ingestion of adequate amounts of fluid, particularly with the bedtime dose, is recommended to reduce the risk of esophageal irritation and ulceration by tetracycline hydrochloride.
If a dose is missed, patients should continue the normal dosing schedule until medication is gone. Patients should not take double doses. If more than 4 doses are missed, the prescriber should be contacted.
3. Dosage Forms and Strengths
Each Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules contains 140 mg of bismuth subcitrate potassium, 125 mg of metronidazole, and a smaller capsule inside containing 125 mg of tetracycline hydrochloride. The capsules are white and opaque, with the APTALIS™ logo printed on the body and "BMT" printed on the cap.
4. Contraindications
4.1 Methoxyflurane
Do not administer methoxyflurane to patients taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. The concurrent use of tetracycline hydrochloride, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, with methoxyflurane has been reported to result in fatal renal toxicity [see Drug Interactions (7.1)].
4.2 Disulfiram
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients who have taken disulfiram within the last two weeks. Psychotic reactions have been reported in alcoholic patients who are using metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, and disulfiram concurrently [see Drug Interactions (7.2)].
4.3 Alcohol
Alcoholic beverages or other products containing propylene glycol should not be consumed during and for at least 3 days after therapy with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. A disulfiram-like reaction (abdominal cramps, nausea, vomiting, headaches, and flushing) may occur due to the interaction between alcohol or propylene glycol and metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride [see Drug Interactions (7.3)].
4.4 Cockayne Syndrome
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients with Cockayne syndrome. Severe irreversible hepatotoxicity/acute liver failure with fatal outcomes have been reported after initiation of metronidazole in patients with Cockayne syndrome [see Adverse Reactions (6.3)].
4.5 Severe Renal Impairment
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients with severe renal impairment. The antianabolic action of the tetracyclines may cause an increase in blood urea nitrogen (BUN) [see Adverse Reactions (6.3)]. In patients with significantly impaired renal function, higher serum concentrations of tetracyclines may lead to azotemia, hyperphosphatemia, and acidosis.
4.6 Pregnancy
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated during pregnancy [see Use in Specific Populations (8.1)].
4.7 Hypersensitivity Reactions
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients with known hypersensitivity (e.g. urticaria, erythematous rash, flushing, and fever) to bismuth subcitrate potassium, metronidazole or other nitroimidazole derivatives, or tetracycline [see Adverse Reactions (6.3)].
5. Warnings and Precautions
5.1 Potential for Carcinogenicity
Metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, has been shown to be carcinogenic in mice and rats. Tumors affecting the liver, lungs, mammary and lymphatic tissues have been detected in several studies of metronidazole in rats and mice, but not hamsters [see Nonclinical Toxicology (13)]. It is unknown whether metronidazole is associated with carcinogenicity in humans.
5.2 Fetal Toxicity
Tetracycline can cause fetal harm when administered to a pregnant woman. Based on animal data, use of drugs of the tetracycline class during the second and third trimester of pregnancy can cause permanent discoloration of the teeth (yellow-gray brown) and possibly inhibit bone development [see Warnings and Precautions (5.4)]. Administration of oral tetracycline to pregnant rats at various doses resulted in yellow fluorescence in teeth and bones in the newborn animals. If bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is used during pregnancy, or if the patient becomes pregnant while taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, advise the patient of the potential risk to the fetus [see Contraindications (4.6) and Use in Specific Populations (8.1)].
5.3 Maternal Toxicity
Tetracycline, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, administered during pregnancy at high doses (> 2 g IV) was associated with rare but serious cases of maternal hepatotoxicity. This syndrome may result in stillborn or premature birth due to maternal pathology [see Contraindications (4.6) and Use in Specific Populations (8.1)].
5.4 Tooth Enamel Discoloration and Hypoplasia
The use of drugs of the tetracycline class during tooth development (last half of pregnancy, infancy, and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term use of the drug, but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, therefore, should not be used in this age group unless other drugs are not likely to be effective or are contraindicated [see Use in Specific Populations (8.4)].
5.5 Central and Peripheral Nervous System Effects
5.6 Development of Potential for Microbial Overgrowth
Known or previously unrecognized candidiasis may present more prominent symptoms during therapy with metronidazole and requires treatment with an antifungal agent. As with other antibacterial drugs, use of tetracycline hydrochloride may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, discontinue bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and institute appropriate therapy.
5.7 Photosensitivity
Photosensitivity, manifested by an exaggerated sunburn reaction, has been observed in patients taking tetracycline [see Adverse Reactions (6.3)]. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs. Instruct patients taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride to avoid exposure to the sun or sun lamps. Discontinue treatment at the first evidence of skin erythema.
5.8 Darkening of the Tongue and/or Black Stool
Bismuth subcitrate potassium may cause temporary and harmless darkening of the tongue and/or black stools, generally reversible within several days after treatment is stopped [see Adverse Reactions (6.1)]. Stool darkening should not be confused with melena.
5.9 Use in Patients with Blood Dyscrasias
Metronidazole is a nitroimidazole, and should be used with care in patients with evidence of or history of blood dyscrasia. A mild leukopenia has been observed during its administration; however, no persistent hematologic abnormalities attributable to metronidazole have been observed in clinical studies. Total and differential leukocyte counts are recommended before and after therapy [see Adverse Reactions (6.3)].
5.10 Increased Drug Plasma Concentrations in Patients with Hepatic Impairment
Patients with hepatic impairment metabolize metronidazole slowly, with resultant accumulation of metronidazole in the plasma. Patients with mild to moderate hepatic impairment should be monitored for metronidazole associated adverse events. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Clinical Pharmacology (12.3)].
5.11 Laboratory Test Interactions
Bismuth absorbs x-rays and may interfere with x-ray diagnostic procedures of the gastrointestinal tract.
Bismuth subcitrate potassium may cause a temporary and harmless darkening of the stool. However, this change does not interfere with standard tests for occult blood.
Metronidazole may interfere with certain types of determinations of serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides, and hexokinase glucose. Values of zero may be observed. All of the assays in which interference has been reported involve enzymatic coupling of the assay to oxidation-reduction of nicotinamide (NAD+ <=> NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7.
5.12 Development of Drug Resistant Bacteria
Prescribing bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.13 Cutaneous Reactions
Skin and subcutaneous disorders including Stevens-Johnson syndrome, toxic epidermal necrolysis and DRESS syndrome (drug rash with eosinophilia and systemic symptoms) have been reported. Discontinue treatment at the first evidence of a cutaneous reaction [see Adverse Reactions (6.2)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride plus omeprazole (OBMT) to eradicate Helicobacter pylori was assessed in an open-label, randomized, active-controlled clinical trial conducted in North America. The duration of treatment was 10 days with 147 patients exposed to bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride plus omeprazole (OBMT) and 152 exposed to control, consisting of omeprazole, amoxicillin, and clarithromycin (OAC). The age of the population in the study ranged from 18 to 75 years, with 59% male patients and 59% Caucasian patients.
Adverse drug reactions were reported in 58% of patients in the OBMT group and 59% of patients in the OAC group. There were no adverse reactions leading to discontinuation of the study during the clinical trial.
Adverse reactions with an incidence of ≥ 5% in OBMT group include abnormal feces, diarrhea, nausea, and headache. Adverse drug reactions with an incidence of ≥ 5% in OAC group include diarrhea, dysgeusia, dyspepsia, nausea and headache.
Table 2 lists adverse reactions with an incidence of ≥ 1%, in either groups (OBMT vs OAC) and in order of decreasing incidence for the OBMT group.
| Preferred Term | OBMT* (n = 147) | OAC† (n = 152) |
|---|---|---|
|
||
| Gastrointestinal disorders | ||
| Abnormal feces‡ | 23 (15.6%) | 7 (4.6%) |
| Nausea | 12 (8.2%) | 14 (9.2%) |
| Diarrhea | 10 (6.8%) | 20 (13.2%) |
| Abdominal Pain | 7 (4.8%) | 2 (1.3%) |
| Dyspepsia | 4 (2.7%) | 10 (6.6%) |
| Constipation | 2 (1.4%) | 5 (3.3%) |
| Dry Mouth | 2 (1.4%) | 1 (0.7%) |
| Flatulence | 0 | 4 (2.6%) |
| Glositis | 0 | 2 (1.3%) |
| General disorders and administration site conditions | ||
| Asthenia | 5 (3.4%) | 2 (1.3%) |
| Infections and infestations | ||
| Vaginal infection | 4 (2.7%) | 3 (2.0%) |
| Nervous system disorders | ||
| Headache | 8 (5.4%) | 8 (5.3%) |
| Dysgeusia | 6 (4.1%) | 18 (11.8%) |
| Dizziness | 4 (2.7%) | 4 (2.6%) |
| Investigations | ||
| Laboratory test abnormal | 3 (2.0%) | 4 (2.6%) |
| Alanine aminotransferase increased | 2 (1.4%) | 0 |
| Aspartate aminotransferase increased | 2 (1.4%) | 0 |
| Renal and urinary disorders | ||
| Urine abnormality | 2 (1.4%) | 0 |
| Skin and subcutaneous tissue disorders | ||
| Rash Maculo-Papular | 2 (1.4%) | 0 |
| Rash | 1 (0.7%) | 3 (2.0%) |
| Pruritus | 0 | 4 (2.6%) |
Adverse reactions with an incidence of <1% for OBMT group are: back pain, vomiting, tongue darkening [see Warnings and Precautions (5.8)], anxiety, gastritis, gastroenteritis, myalgia, chest pain, increased appetite, blood creatine phosphokinase increased, malaise, somnolence, tachycardia, duodenal ulcer, visual disturbance, weight increased.
6.2 Postmarketing Experience
Additionally, the following adverse reactions, presented by system organ class in alphabetical order, have been identified during post approval use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal disorders: abdominal distention, eructation, flatulence
- General disorders and administration site conditions: chest discomfort, fatigue
- Infections and infestations: candidiasis, pseudomembranous colitis (Clostridium difficile colitis)
- Nervous Systems: peripheral neuropathy
- Skin and subcutaneous disorders: Stevens-Johnson syndrome, toxic epidermal necrolysis, DRESS syndrome (drug rash with eosinophilia and systemic symptoms)
7. Drug Interactions
7.1 Methoxyflurane
Do not administer methoxyflurane to patients taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. The concurrent use of tetracycline hydrochloride, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, with methoxyflurane has been reported to result in fatal renal toxicity [see Contraindications (4.1)].
7.2 Disulfiram
Psychotic reactions have been reported in alcoholic patients who are using metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and disulfiram concurrently. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride should not be given to patients who have taken disulfiram within the last two weeks [see Contraindications (4.2)].
7.3 Alcohol
Consumption of alcoholic beverages or administration of other products containing propylene glycol during treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and for at least 3 days afterwards may cause a disulfiram-like reaction (abdominal cramps, nausea, vomiting, headaches, and flushing) due to the interaction between alcohol or propylene glycol and metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. Discontinue alcoholic beverage or other products containing propylene glycol during and for at least 3 days after therapy with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride [see Contraindications (4.3)].
7.4 Oral Contraceptives
Concurrent use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride with oral contraceptive may make oral contraceptives less effective due to an interaction with the tetracycline component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. Breakthrough bleeding has been reported. Women of child-bearing potential should use a different or additional form of contraception while taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride [see Warnings and Precautions (5.14)].
7.5 Anticoagulants
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may alter the anticoagulant effects of warfarin and other oral coumarin anticoagulants. Metronidazole has been reported to potentiate the anticoagulant effect of warfarin, and other oral coumarin anticoagulants, resulting in a prolongation of prothrombin time. Tetracycline has been shown to depress plasma prothrombin activity. Prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored if bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding [see Warnings and Precautions (5.14)].
7.6 Lithium
In patients stabilized on relatively high doses of lithium, short-term use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may cause elevation of serum lithium concentrations and signs of lithium toxicity due to the interaction between metronidazole and lithium. Serum lithium and serum creatinine concentrations should be monitored several days after beginning treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride to detect any increase that may precede clinical symptoms of lithium toxicity [see Warnings and Precautions (5.14)].
7.7 Antacids, Multivitamins, or Dairy Products
The absorption of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may be reduced if administered with antacids containing aluminium, calcium, or magnesium; preparations containing iron, zinc, or sodium bicarbonate; or milk or dairy products due to the interaction between these products and tetracycline. These products should not be consumed concomitantly with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. However, the clinical significance of reduced tetracycline systemic exposure is unknown as the relative contribution of systemic versus local antimicrobial activity against Helicobacter pylori has not been established.
7.8 Busulfan
Metronidazole has been reported to increase plasma concentrations of busulfan, which can result in an increased risk for serious busulfan toxicity. Do not administer bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride concomitantly with busulfan unless the benefit outweighs the risk. If no therapeutic alternatives to bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride are available, and concomitant administration with busulfan is medically needed, monitor for busulfan toxicity and busulfan plasma concentrations and adjust the busulfan dose accordingly [see Warnings and Precautions (5.14)].
7.9 Inhibitors of CYP450 liver enzymes
The simultaneous administration of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and drugs that inhibit microsomal liver enzymes, such as cimetidine, may result in a prolonged half-life and decreased plasma clearance of metronidazole.
7.10 Inducers of CYP450 liver enzymes
The simultaneous administration of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and drugs that induce microsomal liver enzymes, such as phenytoin or phenobarbital, may accelerate the elimination of metronidazole, resulting in reduced plasma concentrations of metronidazole. Impaired clearance of phenytoin has also been reported in this situation. Monitor phenytoin concentrations during treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride.
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride in pediatric patients infected with Helicobacter pylori have not been established.
Tetracycline use in children may cause permanent discoloration of the teeth. Enamel hypoplasia has also been reported. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride should not be used in children up to 8 years of age [see Warnings and Precaution (5.4)].
8.5 Geriatric Use
Clinical studies of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, elderly patients may have a greater frequency of decreased hepatic, renal, or cardiac function, and concomitant diseases or other drug therapies. Bismuth subcitrate potassium, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, additional monitoring may be required [see Contraindications (4.5)].
8.6 Renal Impairment
The antianabolic action of the tetracyclines may cause an increase in blood urea nitrogen (BUN). In patients with severe renal impairment, higher serum concentrations of tetracycline may lead to azotemia, hyperphosphatemia, and acidosis [see Contraindications (4.5)].
8.7 Hepatic Impairment
Patients with severe hepatic disease metabolize metronidazole slowly, with resultant accumulation of metronidazole and its metabolites in plasma. Patients with mild to moderate hepatic impairment should be monitored for metronidazole associated adverse events. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is not recommended in patients with severe hepatic impairment [see Warnings and Precautions (5.10) and Clinical Pharmacology (12.3)].
10. Overdosage
In case of an overdose, patients should contact a physician, poison control center, or emergency room. The available overdosage information for each of the individual components in bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride are summarized below:
11. Bismuth, Metronidazole and Tetracycline Capsules Description
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules are a combination antimicrobial product containing bismuth subcitrate potassium, metronidazole, and tetracycline hydrochloride for oral administration. Each size 0 elongated capsule contains:
- bismuth subcitrate potassium, 140 mg
- metronidazole, 125 mg
- smaller capsule (size 3) containing tetracycline hydrochloride, 125 mg
Tetracycline hydrochloride is encapsulated within a smaller capsule to create a barrier to avoid contact with bismuth subcitrate potassium.
Each Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsule contains the following inactive ingredients: Magnesium Stearate NF, Lactose Monohydrate NF, Talc USP, Gelatin USP, and Titanium Dioxide NF, Printed in red ink.
Bismuth subcitrate potassium is a white or almost white powder. It is a soluble, complex bismuth salt of citric acid. The schematized empirical molecular formula of bismuth subcitrate potassium is Bi (Citrate)2K5∙3 H2O. The equivalent theoretical molecular formula is BiC12H14K5O17. The molecular mass of the theoretical molecular formula of a single unit of bismuth subcitrate potassium is 834.71.
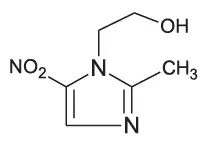
Metronidazole is a white to pale yellow crystalline powder. Metronidazole is 2-methyl-5-nitroimidazole-1-ethanol, with a molecular formula of C6H9N3O3 and the following structural formula:
|
|
|
| Molecular weight: 171.2 | |
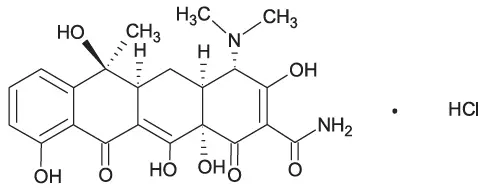
Tetracycline hydrochloride is a yellow, odorless, crystalline powder. Tetracycline hydrochloride is stable in air, but exposure to strong sunlight causes it to darken. Tetracycline hydrochloride is (4S,4aS,5aS,6S,12aS)-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-penta-hydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide hydrochloride, with a molecular formula of C22H24N2O8∙HCl and the following structural formula:
|
|
|
| Molecular weight: 480.90 | |
12. Bismuth, Metronidazole and Tetracycline Capsules - Clinical Pharmacology
12.1 Mechanism of Action
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is a combination of antibacterial agents (metronidazole and tetracycline hydrochloride) and bismuth subcitrate potassium [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetics of the individual components of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride are summarized below. In addition, two studies on bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride were conducted to determine the effect of co-administration on the pharmacokinetics of the components.
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules
A comparative bioavailability study of metronidazole (375 mg), tetracycline hydrochloride (375 mg) and bismuth subcitrate potassium (420 mg, equivalent to 120 mg Bi2O3) administered as bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride or as 3 separate capsule formulations administered simultaneously was conducted in healthy male volunteers. The pharmacokinetic parameters for the individual drugs, when administered as separate capsule formulations or as bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, are similar as shown in Table 3.
| Cmax
(ng/mL) (%C.V.*) | AUCT
(ng ∙ h/mL) (%C.V.*) | AUC∞
(ng ∙ h/mL) (%C.V.*) |
||
|---|---|---|---|---|
|
||||
| Metronidazole | Metronidazole Capsule | 9044 (20) | 80289 (15) | 81849 (16) |
| Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride† | 8666.3 (22) | 83018 (17) | 84413 (17) | |
| Tetracycline | Tetracycline Capsules | 748.0 (40) | 9544 (55) | 9864 (53) |
| Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride† | 774 (47) | 9674 (50) | 9987 (49) | |
| Bismuth | Bismuth Capsule | 22 (123) | 47 (129) | 65.4 (113) |
| Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride† | 17 (202) | 43 (191) | 57 (178) | |
Effect of Food on the Bioavailability of Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride
The pharmacokinetic parameters for metronidazole, tetracycline hydrochloride and bismuth were also determined when bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride was administered under fasting and fed conditions, as shown in Table 4. Food reduced the systemic absorption of all three bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride components, with AUC values for metronidazole, tetracycline hydrochloride and bismuth being reduced by 6%, 34% and 60%, respectively. Reduction in the absorption of all three bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride components in the presence of food is not considered to be clinically significant. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride should be given after meals and at bedtime, in combination with omeprazole twice a day.
| FED | FASTED | |||||
|---|---|---|---|---|---|---|
| metronidazole | tetracycline | bismuth | metronidazole | tetracycline | bismuth | |
|
||||||
| Cmax (ng/mL) (%C.V.) | 6835.0 (13) | 515.8 (36) | 1.7 (61) | 8666.3 (22) | 773.8 (47) | 16.7 (202) |
| Tmax (hours)†
(range) | 3.0 (1.3 - 4.0) | 4.0 (2.5 - 5.0) | 3.5 (0.8 - 6.0) | 0.75 (0.5 - 3.5) | 3.3 (1.3 - 5.0) | 0.6 (0.5 - 1.7) |
| AUC∞
(ng ∙ h/mL) (%C.V.) | 79225.6 (18) | 5840.1 (312) | 18.4 (116) | 84413.6 (17) | 9986.7 (49) | 56.5 (178) |
Effect of Omeprazole on the Bioavailability of Bismuth
The effect of omeprazole on bismuth absorption was assessed in 34 healthy volunteers given bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride (four times daily) with or without omeprazole (20 mg twice daily) for 6 days. In the presence of omeprazole, the extent of absorption of bismuth from bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride was significantly increased, compared to when no omeprazole was given (Table 5). Concentration-dependent neurotoxicity is associated with long-term use of bismuth and not likely to occur with short-term administration or at steady state concentrations below 50 ng/mL. One subject transiently achieved a maximum bismuth concentration (Cmax) higher than 50 ng/mL (73 ng/mL) following multiple dosing of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride with omeprazole. The patient did not exhibit symptoms of neurotoxicity during the study. There is no clinical evidence to suggest that short-term exposure to bismuth Cmax concentrations above 50 ng/mL is associated with neurotoxicity.
| Parameter | Without omeprazole | With omeprazole | ||
|---|---|---|---|---|
| Mean | %C.V.† | Mean | %C.V.† | |
|
||||
| Cmax (ng/mL) | 8.1 | 84 | 25.5 | 69 |
| AUCT (ng ∙ h/mL) | 48.5 | 28 | 140.9 | 42 |
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
An open-label, parallel group, active-controlled, multicenter study in Helicobacter pylori positive patients with current duodenal ulcer or a history of duodenal ulcer disease was conducted in the United States and Canada (the North American Study).
Patients were randomized to one of the following 10-day treatment regimens:
- Three (3) Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules four times daily, after meals and at bedtime plus 20 mg omeprazole twice a day after the morning and evening meals (OBMT).
- Clarithromycin 500 mg plus 1000 mg amoxicillin plus 20 mg omeprazole twice a day before the morning and evening meals (OAC).
H. pylori eradication rates, defined as two negative 13C-urea breath tests performed at 4 and 8 weeks post-therapy are shown in Table 6 for OBMT and OAC. The eradication rates for both groups were found to be similar using either the Per Protocol (PP) or Modified Intent-to-Treat (MITT) populations.
| Treatment Group | Difference | ||
|---|---|---|---|
| OBMT* | OAC† ‡ | ||
|
|||
| Per Protocol§ | 92.5% [87.8, 97.2] (n=120) | 85.7% [76.9, 91.8] (n=126) | 6.8% [-0.9, 14.5] |
| Modified Intent-to-Treat¶ | 87.7% [82.2, 93.2] (n=138) | 83.2% [77.0, 89.5] (n=137) | 4.5% [-3.9, 12.8] |
16. How is Bismuth, Metronidazole and Tetracycline Capsules supplied
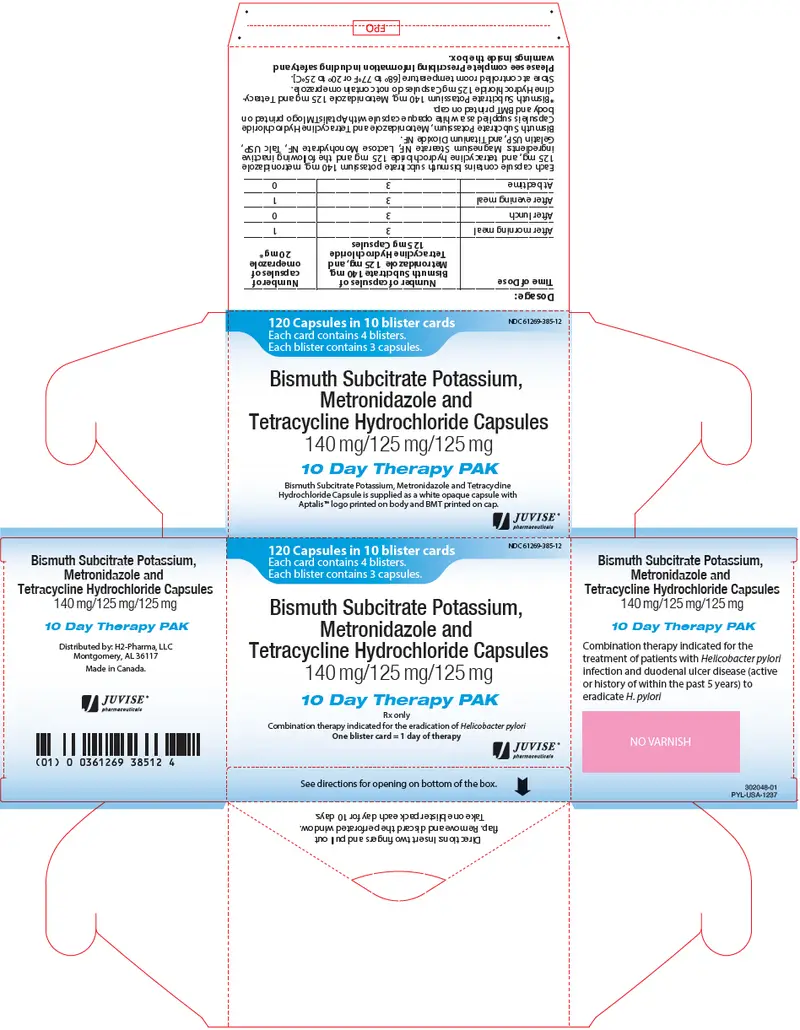
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules are supplied as a white opaque capsule containing 140 mg bismuth subcitrate potassium, 125 mg metronidazole, and 125 mg tetracycline hydrochloride, with the APTALIS™ logo printed on the body and "BMT" printed on the cap. Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules are supplied as the 10 day Therapy pack containing 10 blister cards, with each card containing 12 Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules for a total of 120 capsules.
NDC Number: 61269-385-12, Blister pack of 120.
| BISMUTH SUBCITRATE POTASSIUM, METRONIDAZOLE, AND TETRACYCLINE HYDROCHLORIDE
bismuth subcitrate potassium, metronidazole, and tetracycline hydrochloride capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - H2-Pharma, LLC (028473634) |