Drug Detail:Blincyto (Blinatumomab [ blin-a-toom-oh-mab ])
Drug Class: CD19 monoclonal antibodies
Highlights of Prescribing Information
BLINCYTO® (blinatumomab) for injection, for intravenous use
Initial U.S. Approval: 2014
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO and treat with corticosteroids as recommended. (2.3, 5.1)
- Neurological toxicities, which may be severe, life-threatening, or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO as recommended. (2.3, 5.2)
Recent Major Changes
| Indications and Usage (1.1) | 6/2023 |
| Dosage and Administration (2.6) | 6/2023 |
| Warnings and Precautions, Benzyl Alcohol Toxicity in Neonates (5.12) | 6/2023 |
| Warnings and Precautions, Embryo-Fetal Toxicity (5.13) | 6/2023 |
Indications and Usage for Blincyto
BLINCYTO is a bispecific CD19-directed CD3 T-cell engager indicated for the treatment of adult and pediatric patients with:
- CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1%. (1.1)
- Relapsed or refractory CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). (1.2)
Blincyto Dosage and Administration
-
For the treatment of MRD-positive B-cell Precursor ALL
- -
- See Full Prescribing Information for recommended dose by patient weight and schedule. (2.1)
- -
- Hospitalization is recommended for the first 3 days of the first cycle and the first 2 days of the second cycle. (2.1)
- -
- Premedicate with prednisone or equivalent dexamethasone. (2.1)
-
For the treatment of Relapsed or Refractory B-cell Precursor ALL
- -
- See Full Prescribing Information for recommended dose by patient weight and schedule. (2.2)
- -
- Hospitalization is recommended for the first 9 days of the first cycle and the first 2 days of the second cycle. (2.2)
- -
- Premedicate with dexamethasone. (2.2)
- Refer to Full Prescribing Information for important preparation and administration information. (2.4, 2.5, 2.6)
- Administer as a continuous intravenous infusion at a constant flow rate using an infusion pump. (2.5, 2.6)
- -
- See Section 2.5 for infusion over 24 hours or 48 hours.
- -
- See Section 2.6 for infusion over 7 days using Bacteriostatic 0.9% Sodium Chloride Injection, USP (containing 0.9% benzyl alcohol).
Dosage Forms and Strengths
For injection: 35 mcg of lyophilized powder in a single-dose vial for reconstitution. (3)
Contraindications
Known hypersensitivity to blinatumomab or to any component of the product formulation. (4)
Warnings and Precautions
- Infections: Monitor patients for signs or symptoms; treat appropriately. (5.3)
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while BLINCYTO is being administered. (5.6)
- Pancreatitis: Evaluate patients who develop signs and symptoms of pancreatitis. Management of pancreatitis may require either temporary interruption or discontinuation of BLINCYTO. (5.8)
- Preparation and Administration Errors: Strictly follow instructions for preparation (including admixing) and administration. (5.10)
- Benzyl Alcohol Toxicity in Neonates: Use BLINCYTO prepared with preservative-free saline for neonates. (5.12, 8.4)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. (5.13, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 20%) are pyrexia, infusion-related reactions, infections (pathogen unspecified), headache, neutropenia, anemia, and thrombocytopenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Inc. at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2023
Related/similar drugs
methotrexate, imatinib, mercaptopurine, doxorubicin, Gleevec, SprycelFull Prescribing Information
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES
- Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO and treat with corticosteroids as recommended [see Dosage and Administration (2.3), Warnings and Precautions (5.1)].
- Neurological toxicities, which may be severe, life-threatening, or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO as recommended [see Dosage and Administration (2.3), Warnings and Precautions (5.2)].
1. Indications and Usage for Blincyto
2. Blincyto Dosage and Administration
2.1 Treatment of MRD-positive B-cell Precursor ALL
- A treatment course consists of 1 cycle of BLINCYTO for induction followed by up to 3 additional cycles for consolidation.
- A single cycle of treatment of BLINCYTO induction or consolidation consists of 28 days of continuous intravenous infusion followed by a 14-day treatment-free interval (total 42 days).
- See Table 1 for the recommended dose by patient weight and schedule. Patients weighing 45 kg or more receive a fixed-dose. For patients weighing less than 45 kg, the dose is calculated using the patient's body surface area (BSA).
| Cycle | Patients Weighing 45 kg or More (Fixed-dose) | Patients Weighing Less Than 45 kg (BSA-based dose) |
|---|---|---|
| Induction Cycle 1 | ||
| Days 1-28 | 28 mcg/day | 15 mcg/m2/day (not to exceed 28 mcg/day) |
| Days 29-42 | 14-day treatment-free interval | 14-day treatment-free interval |
| Consolidation Cycles 2-4
| ||
| Days 1-28 | 28 mcg/day | 15 mcg/m2/day (not to exceed 28 mcg/day) |
| Days 29-42 | 14-day treatment-free interval | 14-day treatment-free interval |
- Hospitalization is recommended for the first 3 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and re-initiations (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended.
- Premedicate with prednisone or equivalent for MRD-positive B-cell Precursor ALL
- For adult patients, premedicate with prednisone 100 mg intravenously or equivalent (e.g., dexamethasone 16 mg) 1 hour prior to the first dose of BLINCYTO in each cycle.
- For pediatric patients, premedicate with 5 mg/m2 of dexamethasone, to a maximum dose of 20 mg, prior to the first dose of BLINCYTO in the first cycle and when restarting an infusion after an interruption of 4 or more hours in the first cycle.
- For administration of BLINCYTO:
- See Section 2.5 for infusion over 24 hours or 48 hours.
- See Section 2.6 for infusion over 7 days using Bacteriostatic 0.9% Sodium Chloride Injection, USP (containing 0.9% benzyl alcohol).
2.2 Treatment of Relapsed or Refractory B-cell Precursor ALL
- A treatment course consists of up to 2 cycles of BLINCYTO for induction followed by 3 additional cycles for consolidation and up to 4 additional cycles of continued therapy.
- A single cycle of treatment of BLINCYTO induction or consolidation consists of 28 days of continuous intravenous infusion followed by a 14-day treatment-free interval (total 42 days).
- A single cycle of treatment of BLINCYTO continued therapy consists of 28 days of continuous intravenous infusion followed by a 56-day treatment-free interval (total 84 days).
- See Table 2 for the recommended dose by patient weight and schedule. Patients weighing 45 kg or more receive a fixed-dose and for patients weighing less than 45 kg, the dose is calculated using the patient's BSA.
| Cycle | Patients Weighing 45 kg or More (Fixed-dose) | Patients Weighing Less Than 45 kg (BSA-based dose) |
|---|---|---|
| Induction Cycle 1 | ||
| Days 1-7 | 9 mcg/day | 5 mcg/m2/day (not to exceed 9 mcg/day) |
| Days 8-28 | 28 mcg/day | 15 mcg/m2/day (not to exceed 28 mcg/day) |
| Days 29-42 | 14-day treatment-free interval | 14-day treatment-free interval |
| Induction Cycle 2
| ||
| Days 1-28 | 28 mcg/day | 15 mcg/m2/day (not to exceed 28 mcg/day) |
| Days 29-42 | 14-day treatment-free interval | 14-day treatment-free interval |
| Consolidation Cycles 3-5
| ||
| Days 1-28 | 28 mcg/day | 15 mcg/m2/day (not to exceed 28 mcg/day) |
| Days 29-42 | 14-day treatment-free interval | 14-day treatment-free interval |
| Continued Therapy Cycles 6-9 | ||
| Days 1-28 | 28 mcg/day | 15 mcg/m2/day (not to exceed 28 mcg/day) |
| Days 29-84 | 56-day treatment-free interval | 56-day treatment-free interval |
- Hospitalization is recommended for the first 9 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and re-initiation (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended.
- Premedicate with dexamethasone:
- For adult patients, premedicate with 20 mg of dexamethasone 1 hour prior to the first dose of BLINCYTO of each cycle, prior to a step dose (such as Cycle 1 Day 8), and when restarting an infusion after an interruption of 4 or more hours.
- For pediatric patients, premedicate with 5 mg/m2 of dexamethasone, to a maximum dose of 20 mg, prior to the first dose of BLINCYTO in the first cycle, prior to a step dose (such as Cycle 1 Day 8), and when restarting an infusion after an interruption of 4 or more hours in the first cycle.
- For administration of BLINCYTO:
- See Section 2.5 for infusion over 24 hours or 48 hours.
- See Section 2.6 for infusion over 7 days using Bacteriostatic 0.9% Sodium Chloride Injection, USP (containing 0.9% benzyl alcohol).
2.3 Dosage Modifications for Adverse Reactions
If the interruption after an adverse reaction is no longer than 7 days, continue the same cycle to a total of 28 days of infusion inclusive of days before and after the interruption in that cycle. If an interruption due to an adverse reaction is longer than 7 days, start a new cycle.
| Adverse Reaction | Grade* | Patients Weighing 45 kg or More | Patients Weighing Less Than 45 kg |
|---|---|---|---|
|
|||
| Cytokine Release Syndrome (CRS) | Grade 3 |
|
|
| Grade 4 | Discontinue BLINCYTO permanently. Administer dexamethasone as instructed for Grade 3 CRS. | ||
| Neurological Toxicity | Seizure | Discontinue BLINCYTO permanently if more than one seizure occurs. | |
| Grade 3 | Withhold BLINCYTO until no more than Grade 1 (mild) and for at least 3 days, then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the adverse reaction does not recur. If the adverse reaction occurred at 9 mcg/day, or if the adverse reaction takes more than 7 days to resolve, discontinue BLINCYTO permanently. | Withhold BLINCYTO until no more than Grade 1 (mild) and for at least 3 days, then restart BLINCYTO at 5 mcg/m2/day. Escalate to 15 mcg/m2/day after 7 days if the adverse reaction does not recur. If the adverse reaction occurred at 5 mcg/m2/day, or if the adverse reaction takes more than 7 days to resolve, discontinue BLINCYTO permanently. | |
| Grade 4 | Discontinue BLINCYTO permanently. | ||
| Other Clinically Relevant Adverse Reactions | Grade 3 | Withhold BLINCYTO until no more than Grade 1 (mild), then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the adverse reaction does not recur. If the adverse reaction takes more than 14 days to resolve, discontinue BLINCYTO permanently. | Withhold BLINCYTO until no more than Grade 1 (mild), then restart BLINCYTO at 5 mcg/m2/day. Escalate to 15 mcg/m2/day after 7 days if the adverse reaction does not recur. If the adverse reaction takes more than 14 days to resolve, discontinue BLINCYTO permanently. |
| Grade 4 | Consider discontinuing BLINCYTO permanently. | ||
2.4 Preparation
It is very important that the instructions for preparation (including admixing) and administration provided in this section are strictly followed to minimize medication errors (including underdose and overdose) [see Warnings and Precautions (5.10)].
BLINCYTO can be infused over 24 hours (preservative-free), 48 hours (preservative-free), or 7 days (with preservative). The choice between these options for the infusion duration should be made by the treating healthcare provider considering the frequency of the infusion bag changes and the weight of the patient.
For preparation, reconstitution, and administration of BLINCYTO:
- See Section 2.5 for infusion over 24 hours or 48 hours.
- See Section 2.6 for infusion over 7 days using Bacteriostatic 0.9% Sodium Chloride Injection, USP (containing 0.9% benzyl alcohol).
Call 1-800-77-AMGEN (1-800-772-6436) if you have questions about the reconstitution and preparation of BLINCYTO.
2.5 Preparation and Administration of BLINCYTO as a 24-Hour or 48-Hour Infusion
Reconstitute BLINCYTO with preservative-free Sterile Water for Injection, USP. Do not reconstitute BLINCYTO vials with the IV Solution Stabilizer.
To prime the intravenous tubing, use only the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion. Do not prime with 0.9% Sodium Chloride Injection, USP.
2.6 Preparation and Administration of BLINCYTO as a 7-Day Infusion using Bacteriostatic 0.9% Sodium Chloride Injection, USP (Preservative)
Use preservative-free Sterile Water for Injection to reconstitute BLINCYTO. Do not reconstitute BLINCYTO vials with the IV Solution Stabilizer.
Do not use an in-line filter with a 7-day infusion bag.
Prime the intravenous tubing only with the solution in the bag containing the FINAL prepared solution for infusion. Do not prime with 0.9% Sodium Chloride Injection, USP.
2.6.2 Preparation of BLINCYTO Infusion Bag for 7-Day Infusion
Verify the prescribed dose and infusion duration for each BLINCYTO infusion bag. To minimize errors, use the specific volumes described in Table 6 to prepare the BLINCYTO infusion bag.
- Aseptically add 90 mL Bacteriostatic 0.9% Sodium Chloride Injection, USP to the empty intravenous bag.
- Aseptically transfer 2.2 mL IV Solution Stabilizer to the intravenous bag containing Bacteriostatic 0.9% Sodium Chloride Injection, USP. Gently mix the contents of the bag to avoid foaming. Discard the vial containing the unused IV Solution Stabilizer.
- Aseptically transfer the required volume of reconstituted BLINCYTO solution into the intravenous bag containing Bacteriostatic 0.9% Sodium Chloride Injection, USP and IV Solution Stabilizer. Gently mix the contents of the bag to avoid foaming.
- Refer to Table 6 for the specific volume of reconstituted BLINCYTO. Discard the vial containing unused BLINCYTO.
- Aseptically add the required volume of 0.9% Sodium Chloride Injection, USP to the intravenous bag to obtain a final volume of 110 mL. Gently mix the contents of the bag to avoid foaming.
- Refer to Table 6 for the specific volume of 0.9% Sodium Chloride Injection, USP.
- Under aseptic conditions, attach the intravenous tubing to the intravenous bag.
- Ensure that the intravenous tubing is compatible with the infusion pump.
- Do not use an in-line filter for a 7-day bag.
- Remove air from the intravenous bag. This is particularly important for use with an ambulatory infusion pump.
- Prime the intravenous tubing only with the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.
- Store refrigerated at 2°C to 8°C (36°F to 46°F) if not used immediately [see Dosage and Administration (2.7)].
|
|||||
| Bacteriostatic 0.9% Sodium Chloride Injection, USP (starting volume) | 90 mL | ||||
| IV Solution Stabilizer (fixed volume for 7-day infusion) | 2.2 mL | ||||
| Reconstituted BLINCYTO | Specific volume listed below in table | ||||
| Quantity Sufficient (q.s.) with 0.9% Sodium Chloride Injection, USP to a Final Volume of 110 mL | Specific volume listed below in table | ||||
| Infusion Duration | 7 days | ||||
| Infusion Rate | 0.6 mL/hour | ||||
| Patient Weight | Dose | BSA (m2)* | Reconstituted BLINCYTO | Volume of 0.9% Sodium Chloride Injection, USP needed to q.s. to a Final Volume of 110 mL |
|
| Volume | Vials | ||||
| Fixed-Dose | |||||
| 45 kg or More | 28 mcg/day | N/A | 16.8 mL | 6 | 1 mL |
| BSA-Based Dose | |||||
| Less than 45 kg | 15 mcg/m2/day | 1.5 – 1.59 | 14 mL | 5 | 3.8 mL |
| 1.4 – 1.49 | 13.1 mL | 5 | 4.7 mL | ||
| 1.30 – 1.39 | 12.2 mL | 5 | 5.6 mL | ||
| 1.20 – 1.29 | 11.3 mL | 5 | 6.5 mL | ||
| 1.10 – 1.19 | 10.4 mL | 4 | 7.4 mL | ||
| 1 – 1.09 | 9.5 mL | 4 | 8.3 mL | ||
| 0.9 – 0.99 | 8.6 mL | 4 | 9.2 mL | ||
| 0.8 – 0.89 | 7.7 mL | 3 | 10.1 mL | ||
| 0.7 – 0.79 | 6.8 mL | 3 | 11 mL | ||
| 0.6 – 0.69 | 5.9 mL | 3 | 11.9 mL | ||
| 0.5 – 0.59 | 5 mL | 2 | 12.8 mL | ||
| 0.4 – 0.49 | 4.1 mL | 2 | 13.7 mL | ||
2.6.3 Administration of BLINCYTO as a 7-Day Infusion
- Administer BLINCYTO as a continuous intravenous infusion at a constant flow rate using an infusion pump. The pump should be programmable, lockable, non-elastomeric, and have an alarm.
- The final volume of infusion solution (110 mL) will be more than the volume administered to the patient (100 mL) to account for the priming of the intravenous tubing and to ensure that the patient will receive the full dose of BLINCYTO.
- Do not use an in-line filter for a 7-day bag.
- Infuse prepared BLINCYTO final infusion solution according to the instructions on the pharmacy label on the prepared bag at an infusion rate of 0.6 mL/hour for a duration of 7 days.
- Important Note: Do not flush the BLINCYTO infusion line or intravenous catheter, especially when changing infusion bags. Flushing when changing bags or at completion of infusion can result in excess dosage and complications thereof. When administering via a multi-lumen venous catheter, infuse BLINCYTO through a dedicated lumen.
- At the end of the infusion, dispose of any unused BLINCYTO solution in the intravenous bag and intravenous tubing in accordance with local requirements.
2.7 Storage of Reconstituted BLINCYTO
The information in Table 7 indicates the storage time for the reconstituted BLINCYTO vial and prepared infusion bag.
| Maximum Storage Time | ||
|---|---|---|
| Room Temperature 23°C to 27°C (73°F to 81°F) | Refrigerated 2°C to 8°C (36°F to 46°F) |
|
|
||
| Reconstituted BLINCYTO Vial | 4 hours | 24 hours |
| Prepared BLINCYTO Infusion Bag (Preservative-free) | 48 hours* | 8 days |
| Prepared BLINCYTO Infusion Bag (with Preservative) | 7 days* | 14 days |
3. Dosage Forms and Strengths
For injection: 35 mcg of white to off-white lyophilized powder in a single-dose vial for reconstitution.
4. Contraindications
BLINCYTO is contraindicated in patients with known hypersensitivity to blinatumomab or to any component of the product formulation.
5. Warnings and Precautions
5.1 Cytokine Release Syndrome
Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. The median time to onset of CRS was 2 days after the start of infusion and the median time to resolution of CRS was 5 days among cases that resolved. Manifestations of CRS include fever, headache, nausea, asthenia, hypotension, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased total bilirubin, and disseminated intravascular coagulation (DIC). The manifestations of CRS after treatment with BLINCYTO overlap with those of infusion reactions, capillary leak syndrome (CLS), and hemophagocytic histiocytosis/macrophage activation syndrome (MAS). Using all of these terms to define CRS in clinical trials of BLINCYTO, CRS was reported in 15% of patients with relapsed or refractory ALL and in 7% of patients with MRD-positive ALL [see Adverse Reactions (6.1)].
Monitor patients for signs or symptoms of these events. Advise outpatients on BLINCYTO to contact their healthcare professional for signs and symptoms associated with CRS. If severe CRS occurs, interrupt BLINCYTO until CRS resolves. Discontinue BLINCYTO permanently if life-threatening CRS occurs. Administer corticosteroids for severe or life-threatening CRS [see Dosage and Administration (2.3)].
5.2 Neurological Toxicities
In patients with ALL receiving BLINCYTO in clinical studies, neurological toxicities have occurred in approximately 65% of patients [see Adverse Reactions (6.1)]. Among patients that experienced a neurologic event, the median time to the first event was within the first 2 weeks of BLINCYTO treatment and the majority of events resolved. The most common (≥ 10%) manifestations of neurological toxicity were headache, and tremor; the neurological toxicity profile varied by age group [see Use in Specific Populations (8.4, 8.5)]. Grade 3 or higher (severe, life-threatening, or fatal) neurological toxicities following initiation of BLINCYTO administration occurred in approximately 13% of patients and included encephalopathy, convulsions, speech disorders, disturbances in consciousness, confusion and disorientation, and coordination and balance disorders. Manifestations of neurological toxicity included cranial nerve disorders. The majority of neurologic events resolved following interruption of BLINCYTO, but some resulted in treatment discontinuation.
There is limited experience with BLINCYTO in patients with active ALL in the central nervous system (CNS) or a history of neurologic events. Patients with a history or presence of clinically relevant CNS pathology were excluded from clinical studies.
Monitor patients receiving BLINCYTO for signs and symptoms of neurological toxicities. Advise outpatients on BLINCYTO to contact their healthcare professional if they develop signs or symptoms of neurological toxicities. Interrupt or discontinue BLINCYTO as recommended [see Dosage and Administration (2.3)].
5.3 Infections
In patients with ALL receiving BLINCYTO in clinical studies, serious infections such as sepsis, pneumonia, bacteremia, opportunistic infections, and catheter-site infections were observed in approximately 25% of patients, some of which were life-threatening or fatal [see Adverse Reactions (6.1)]. As appropriate, administer prophylactic antibiotics and employ surveillance testing during treatment with BLINCYTO. Monitor patients for signs and symptoms of infection and treat appropriately.
5.4 Tumor Lysis Syndrome
Tumor lysis syndrome (TLS), which may be life-threatening or fatal, has been observed in patients receiving BLINCYTO [see Adverse Reactions (6.1)]. Appropriate prophylactic measures, including pretreatment nontoxic cytoreduction and on-treatment hydration, should be used for the prevention of TLS during BLINCYTO treatment. Monitor for signs or symptoms of TLS. Management of these events may require either temporary interruption or discontinuation of BLINCYTO [see Dosage and Administration (2.3)].
5.5 Neutropenia and Febrile Neutropenia
Neutropenia and febrile neutropenia, including life-threatening cases, have been observed in patients receiving BLINCYTO [see Adverse Reactions (6.1)]. Monitor laboratory parameters (including, but not limited to, white blood cell count and absolute neutrophil count) during BLINCYTO infusion. Interrupt BLINCYTO if prolonged neutropenia occurs.
5.6 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including seizures, patients receiving BLINCYTO are at risk for loss of consciousness [see Warnings and Precautions (5.2)]. Advise patients to refrain from driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while BLINCYTO is being administered.
5.7 Elevated Liver Enzymes
Treatment with BLINCYTO was associated with transient elevations in liver enzymes [see Adverse Reactions (6.1)]. In patients with ALL receiving BLINCYTO in clinical studies, the median time to onset of elevated liver enzymes was 3 days.
The majority of these transient elevations in liver enzymes were observed in the setting of CRS. For the events that were observed outside the setting of CRS, the median time to onset was 19 days. Grade 3 or greater elevations in liver enzymes occurred in approximately 7% of patients outside the setting of CRS and resulted in treatment discontinuation in less than 1% of patients.
Monitor alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total blood bilirubin prior to the start of and during BLINCYTO treatment. Interrupt BLINCYTO if the transaminases rise to greater than 5 times the upper limit of normal or if total bilirubin rises to more than 3 times the upper limit of normal.
5.8 Pancreatitis
Fatal pancreatitis has been reported in patients receiving BLINCYTO in combination with dexamethasone in clinical studies and the postmarketing setting [see Adverse Reactions (6.3)].
Evaluate patients who develop signs and symptoms of pancreatitis. Management of pancreatitis may require either temporary interruption or discontinuation of BLINCYTO and dexamethasone [see Dosage and Administration (2.3)].
5.9 Leukoencephalopathy
Cranial magnetic resonance imaging (MRI) changes showing leukoencephalopathy have been observed in patients receiving BLINCYTO, especially in patients with prior treatment with cranial irradiation and antileukemic chemotherapy (including systemic high-dose methotrexate or intrathecal cytarabine). The clinical significance of these imaging changes is unknown.
5.10 Preparation and Administration Errors
Preparation and administration errors have occurred with BLINCYTO treatment. Follow instructions for preparation (including admixing) and administration strictly to minimize medication errors (including underdose and overdose) [see Dosage and Administration (2.4)].
5.11 Immunization
The safety of immunization with live viral vaccines during or following BLINCYTO therapy has not been studied. Vaccination with live virus vaccines is not recommended for at least 2 weeks prior to the start of BLINCYTO treatment, during treatment, and until immune recovery following last cycle of BLINCYTO.
5.12 Benzyl Alcohol Toxicity in Neonates
Serious adverse reactions, including fatal reactions and the "gasping syndrome," have been reported in very low birth weight (VLBW) neonates born weighing less than 1500 g, and early preterm neonates (infants born less than 34 weeks gestational age) who received intravenous drugs containing benzyl alcohol as a preservative. Early preterm VLBW neonates may be more likely to develop these reactions, because they may be less able to metabolize benzyl alcohol [see Use in Specific Populations (8.4)].
Use the preservative-free preparations of BLINCYTO where possible in neonates. When prescribing BLINCYTO (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO (with preservative), other products containing benzyl alcohol or other excipients (e.g., ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway.
Monitor neonatal patients receiving BLINCYTO (with preservative) for new or worsening metabolic acidosis. The minimum amount of benzyl alcohol at which serious adverse reactions may occur in neonates is not known. The BLINCYTO 7-Day bag (with preservative) contains 7.4 mg of benzyl alcohol per mL [see Use in Specific Populations (8.4)].
5.13 Embryo-Fetal Toxicity
Based on its mechanism of action, BLINCYTO may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with BLINCYTO and for 48 hours after the last dose [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Neurological Toxicities [see Warnings and Precautions (5.2)]
- Infections [see Warnings and Precautions (5.3)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.4)]
- Neutropenia and Febrile Neutropenia [see Warnings and Precautions (5.5)]
- Effects on Ability to Drive and Use Machines [see Warnings and Precautions (5.6)]
- Elevated Liver Enzymes [see Warnings and Precautions (5.7)]
- Pancreatitis [see Warnings and Precautions (5.8)]
- Leukoencephalopathy [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of BLINCYTO in patients with MRD-positive B cell precursor ALL (n = 137) and Philadelphia chromosome-negative relapsed or refractory B cell precursor ALL (n = 267) was evaluated in three clinical studies. The most common adverse reactions (≥ 20%) in this pooled population were pyrexia, infusion-related reactions, infections (pathogen unspecified), headache, neutropenia, anemia, and thrombocytopenia.
MRD-positive B-cell Precursor ALL
The safety of BLINCYTO in patients with MRD-positive B-cell precursor ALL was evaluated in two single-arm clinical studies in which 137 patients were treated with BLINCYTO. The median age of the study population was 45 years (range: 18 to 77 years).
The most common adverse reactions (≥ 20%) were pyrexia, infusion-related reactions, headache, infections (pathogen unspecified), tremor, and chills. Serious adverse reactions were reported in 61% of patients. The most common serious adverse reactions (≥ 2%) included pyrexia, tremor, encephalopathy, aphasia, lymphopenia, neutropenia, overdose, device related infection, seizure, and staphylococcal infection. Adverse reactions of Grade 3 or higher were reported in 64% of patients. Discontinuation of therapy due to adverse reactions occurred in 17% of patients; neurologic events were the most frequently reported reasons for discontinuation. There were 2 fatal adverse reactions that occurred within 30 days of the end of BLINCYTO treatment (atypical pneumonia and subdural hemorrhage).
Table 8 summarizes the adverse reactions occurring at a ≥ 10% incidence for any grade or ≥ 5% incidence for Grade 3 or higher.
| Adverse Reaction | BLINCYTO (N = 137) |
|
|---|---|---|
| Any Grade*
n (%) | Grade ≥ 3*
n (%) |
|
|
||
| Blood and lymphatic system disorders | ||
| Neutropenia† | 21 (15) | 21 (15) |
| Leukopenia‡ | 19 (14) | 13 (9) |
| Thrombocytopenia§ | 14 (10) | 8 (6) |
| Cardiac disorders | ||
| Arrhythmia¶ | 17 (12) | 3 (2) |
| General disorders and administration site conditions | ||
| Pyrexia# | 125 (91) | 9 (7) |
| Chills | 39 (28) | 0 (0) |
| Infections and infestations | ||
| Infections - pathogen unspecified | 53 (39) | 11 (8) |
| Injury, poisoning and procedural complications | ||
| Infusion-related reactionÞ | 105 (77) | 7 (5) |
| Investigations | ||
| Decreased immunoglobulinsß | 25 (18) | 7 (5) |
| Weight increased | 14 (10) | 1 (< 1) |
| Hypertransaminasemiaà | 13 (9) | 9 (7) |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 16 (12) | 1 (< 1) |
| Nervous system disorders | ||
| Headache | 54 (39) | 5 (4) |
| Tremorè | 43 (31) | 6 (4) |
| Aphasia | 16 (12) | 1 (< 1) |
| Dizziness | 14 (10) | 1 (< 1) |
| Encephalopathyð | 14 (10) | 6 (4) |
| Psychiatric disorders | ||
| Insomniaø | 24 (18) | 1 (< 1) |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | 18 (13) | 0 (0) |
| Skin and subcutaneous tissue disorders | ||
| Rashý | 22 (16) | 1 (< 1) |
| Vascular disorders | ||
| Hypotension | 19 (14) | 1 (< 1) |
Additional adverse reactions in adult patients with MRD-positive ALL that did not meet the threshold criteria for inclusion in Table 8 were:
Blood and lymphatic system disorders: anemia
General disorders and administration site conditions: edema peripheral, pain, and chest pain (includes chest pain and musculoskeletal chest pain)
Hepatobiliary disorders: blood bilirubin increased
Immune system disorders: hypersensitivity and cytokine release syndrome
Infections and infestations: viral infectious disorders, bacterial infectious disorders, and fungal infectious disorders
Injury, poisoning and procedural complications: medication error and overdose (includes overdose and accidental overdose)
Investigations: blood alkaline phosphatase increased
Musculoskeletal and connective tissue disorders: pain in extremity and bone pain
Nervous system disorders: seizure (includes seizure and generalized tonic-clonic seizure), speech disorder, and hypoesthesia
Psychiatric disorders: confusional state, disorientation, and depression
Respiratory, thoracic and mediastinal disorders: dyspnea and productive cough
Vascular disorders: hypertension (includes blood pressure increased and hypertension) flushing (includes flushing and hot flush), and capillary leak syndrome
Philadelphia Chromosome-negative Relapsed or Refractory B-cell Precursor ALL
The safety of BLINCYTO was evaluated in a randomized, open-label, active-controlled clinical study (TOWER Study) in which 376 patients with Philadelphia chromosome-negative relapsed or refractory B-cell precursor ALL were treated with BLINCYTO (n = 267) or standard of care (SOC) chemotherapy (n = 109). The median age of BLINCYTO-treated patients was 37 years (range: 18 to 80 years), 60% were male, 84% were White, 7% Asian, 2% were Black or African American, 2% were American Indian or Alaska Native, and 5% were Multiple/Other.
The most common adverse reactions (≥ 20%) in the BLINCYTO arm were infections (bacterial and pathogen unspecified), pyrexia, headache, infusion-related reactions, anemia, febrile neutropenia, thrombocytopenia, and neutropenia. Serious adverse reactions were reported in 62% of patients. The most common serious adverse reactions (≥ 2%) included febrile neutropenia, pyrexia, sepsis, pneumonia, overdose, septic shock, CRS, bacterial sepsis, device related infection, and bacteremia. Adverse reactions of Grade 3 or higher were reported in 87% of patients. Discontinuation of therapy due to adverse reactions occurred in 12% of patients treated with BLINCYTO; neurologic events and infections were the most frequently reported reasons for discontinuation of treatment due to an adverse reaction. Fatal adverse events occurred in 16% of patients. The majority of the fatal events were infections.
The adverse reactions occurring at a ≥ 10% incidence for any grade or ≥ 5% incidence for Grade 3 or higher in the BLINCYTO-treated patients in first cycle of therapy are summarized in Table 9.
| Adverse Reaction | BLINCYTO (N = 267) | Standard of Care (SOC) Chemotherapy (N = 109) |
||
|---|---|---|---|---|
| Any Grade*
n (%) | Grade ≥ 3*
n (%) | Any Grade*
n (%) | Grade ≥ 3*
n (%) |
|
|
||||
| Blood and lymphatic system disorders | ||||
| Neutropenia† | 84 (31) | 76 (28) | 67 (61) | 61 (56) |
| Anemia‡ | 68 (25) | 52 (19) | 45 (41) | 37 (34) |
| Thrombocytopenia§ | 57 (21) | 47 (18) | 42 (39) | 40 (37) |
| Leukopenia¶ | 21 (8) | 18 (7) | 9 (8) | 9 (8) |
| Cardiac disorders | ||||
| Arrhythmia# | 37 (14) | 5 (2) | 18 (17) | 0 (0) |
| General disorders and administration site conditions | ||||
| Pyrexia | 147 (55) | 15 (6) | 43 (39) | 4 (4) |
| EdemaÞ | 48 (18) | 3 (1) | 20 (18) | 1 (1) |
| Immune system disorders | ||||
| Cytokine release syndromeß | 37 (14) | 8 (3) | 0 (0) | 0 (0) |
| Infections and infestations | ||||
| Infections - pathogen unspecified | 74 (28) | 40 (15) | 50 (46) | 35 (32) |
| Bacterial infectious disorders | 38 (14) | 19 (7) | 35 (32) | 21 (19) |
| Viral infectious disorders | 30 (11) | 4 (1) | 14 (13) | 0 (0) |
| Fungal infectious disorders | 27 (10) | 13 (5) | 15 (14) | 9 (8) |
| Injury, poisoning and procedural complications | ||||
| Infusion-related reactionà | 79 (30) | 9 (3) | 9 (8) | 1 (1) |
| Investigations | ||||
| Hypertransaminasemiaè | 40 (15) | 22 (8) | 13 (12) | 7 (6) |
| Nervous system disorders | ||||
| Headache | 61 (23) | 1 (< 1) | 30 (28) | 3 (3) |
| Skin and subcutaneous tissue disorders | ||||
| Rashð | 31 (12) | 2 (1) | 21 (19) | 0 (0) |
Selected laboratory abnormalities worsening from baseline Grade 0-2 to treatment-related maximal Grade 3-4 in first cycle of therapy are shown in Table 10.
| BLINCYTO Grade 3 or 4 (%) | SOC Chemotherapy Grade 3 or 4 (%) |
|
|---|---|---|
|
||
| Hematology | ||
| Decreased lymphocyte count | 80 | 83 |
| Decreased white blood cell count | 53 | 97 |
| Decreased hemoglobin | 29 | 43 |
| Decreased neutrophil count | 57 | 68 |
| Decreased platelet count | 47 | 85 |
| Chemistry | ||
| Increased ALT | 11 | 11 |
| Increased bilirubin | 5 | 4 |
| Increased AST | 8 | 4 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of BLINCYTO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Fatal pancreatitis has been reported in patients receiving BLINCYTO in combination with dexamethasone.
7. Drug Interactions
No formal drug interaction studies have been conducted with BLINCYTO. Initiation of BLINCYTO treatment causes transient release of cytokines that may suppress CYP450 enzymes. The highest drug-drug interaction risk is during the first 9 days of the first cycle and the first 2 days of the second cycle in patients who are receiving concomitant CYP450 substrates, particularly those with a narrow therapeutic index. In these patients, monitor for toxicity (e.g., warfarin) or drug concentrations (e.g., cyclosporine). Adjust the dose of the concomitant drug as needed [see Clinical Pharmacology (12.2, 12.3)].
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
BLINCYTO may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.5 Geriatric Use
Of the total number of patients with ALL treated in clinical studies of BLINCYTO, approximately 12% were 65 and over, while 2% were 75 and older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. However, elderly patients experienced a higher rate of serious infections and neurological toxicities, including cognitive disorder, encephalopathy, and confusion [see Warnings and Precautions (5.2, 5.3)].
10. Overdosage
Overdoses have been observed, including one adult patient who received 133-fold the recommended therapeutic dose of BLINCYTO delivered over a short duration.
In the dose evaluation phase of a study in pediatric and adolescent patients with relapsed or refractory B-cell precursor ALL, one patient experienced a fatal cardiac failure event in the setting of life-threatening cytokine release syndrome (CRS) at a 30 mcg/m2/day (higher than the maximum tolerated/recommended) dose [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
Overdoses resulted in adverse reactions, which were consistent with the reactions observed at the recommended dosage and included fever, tremors, and headache. In the event of overdose, interrupt the infusion, monitor the patient for signs of adverse reactions, and provide supportive care [see Warnings and Precautions (5.10)]. Consider re-initiation of BLINCYTO at the recommended dosage when all adverse reactions have resolved and no earlier than 12 hours after interruption of the infusion [see Dosage and Administration (2.1)].
11. Blincyto Description
Blinatumomab is a bispecific CD19-directed CD3 T-cell engager. Blinatumomab is produced in Chinese hamster ovary cells. It consists of 504 amino acids and has a molecular weight of approximately 54 kilodaltons.
Each BLINCYTO package contains 1 vial BLINCYTO and 1 vial IV Solution Stabilizer.
BLINCYTO (blinatumomab) for injection is supplied in a single-dose vial as a sterile, preservative-free, white to off-white lyophilized powder for intravenous use. Each single-dose vial of BLINCYTO contains 35 mcg blinatumomab, citric acid monohydrate (3.35 mg), lysine hydrochloride (23.23 mg), polysorbate 80 (0.64 mg), trehalose dihydrate (95.5 mg), and sodium hydroxide to adjust pH to 7.0. After reconstitution with 3 mL of preservative-free Sterile Water for Injection, USP, the resulting concentration is 12.5 mcg/mL blinatumomab.
IV Solution Stabilizer is supplied in a single-dose vial as a sterile, preservative-free, colorless to slightly yellow, clear solution. Each single-dose vial of IV Solution Stabilizer contains citric acid monohydrate (52.5 mg), lysine hydrochloride (2283.8 mg), polysorbate 80 (10 mg), sodium hydroxide to adjust pH to 7.0, and water for injection.
12. Blincyto - Clinical Pharmacology
12.1 Mechanism of Action
Blinatumomab is a bispecific CD19-directed CD3 T-cell engager that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells. It activates endogenous T cells by connecting CD3 in the T-cell receptor (TCR) complex with CD19 on benign and malignant B cells. Blinatumomab mediates the formation of a synapse between the T-cell and the tumor cell, upregulation of cell adhesion molecules, production of cytolytic proteins, release of inflammatory cytokines, and proliferation of T cells, which result in redirected lysis of CD19+ cells.
12.2 Pharmacodynamics
During the continuous intravenous infusion over 4 weeks, the pharmacodynamic response was characterized by T-cell activation and initial redistribution, reduction in peripheral B cells, and transient cytokine elevation.
Peripheral T-cell redistribution (i.e., T-cell adhesion to blood vessel endothelium and/or transmigration into tissue) occurred after start of BLINCYTO infusion or dose escalation. T-cell counts initially declined within 1 to 2 days and then returned to baseline levels within 7 to 14 days in the majority of patients. Increase of T-cell counts above baseline (T-cell expansion) was observed in few patients.
Peripheral B-cell counts decreased to less than or equal to 10 cells/microliter during the first treatment cycle at doses ≥ 5 mcg/m2/day or ≥ 9 mcg/day in the majority of patients. No recovery of peripheral B-cell counts was observed during the 2-week BLINCYTO-free period between treatment cycles. Incomplete depletion of B cells occurred at doses of 0.5 mcg/m2/day and 1.5 mcg/m2/day and in a few patients at higher doses.
Cytokines including IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, and IFN-γ were measured, and IL-6, IL-10, and IFN-γ were elevated. The highest elevation of cytokines was observed in the first 2 days following start of BLINCYTO infusion. The elevated cytokine levels returned to baseline within 24 to 48 hours during the infusion. In subsequent treatment cycles, cytokine elevation occurred in fewer patients with lesser intensity compared to the initial 48 hours of the first treatment cycle.
12.3 Pharmacokinetics
The pharmacokinetics of blinatumomab appear linear over a dose range from 5 to 90 mcg/m2/day (approximately equivalent to 9 to 162 mcg/day) in adult patients. Following continuous intravenous infusion, the steady-state serum concentration (Css) was achieved within a day and remained stable over time. The increase in mean Css values was approximately proportional to the dose in the range tested. At the clinical doses of 9 mcg/day and 28 mcg/day for the treatment of relapsed or refractory ALL, the mean (SD) Css was 228 (356) pg/mL and 616 (537) pg/mL, respectively. The pharmacokinetics of blinatumomab in adult patients with MRD-positive B-cell precursor ALL was similar to adult patients with relapsed or refractory ALL.
12.6 Immunogenicity
The observed incidence of anti-drug antibody is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibody in the studies described below with the incidence of anti-drug antibodies in other studies, including those of BLINCYTO.
The immunogenicity of BLINCYTO has been evaluated using either an electrochemiluminescence detection technology (ECL) or an enzyme-linked immunosorbent assay (ELISA) screening immunoassay for the detection of binding anti-blinatumomab antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
In clinical studies, less than 2% of patients treated with BLINCYTO tested positive for binding anti-blinatumomab antibodies. Of patients who developed anti-blinatumomab antibodies, 7 out of 9 (78%) had in vitro neutralizing activity. Anti-blinatumomab antibody formation may affect pharmacokinetics of BLINCYTO.
Overall, the totality of clinical evidence supports the finding that anti-blinatumomab antibodies are not suggestive of any clinical impact on the safety or effectiveness of BLINCYTO.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with blinatumomab.
No studies have been conducted to evaluate the effects of blinatumomab on fertility. A murine surrogate molecule had no adverse effects on male and female reproductive organs in a 13-week repeat-dose toxicity study in mice.
14. Clinical Studies
14.1 MRD-positive B-cell Precursor ALL
BLAST Study
The efficacy of BLINCYTO was evaluated in an open-label, multicenter, single-arm study (BLAST Study) [NCT01207388] that included patients who were ≥ 18 years of age, had received at least 3 chemotherapy blocks of standard ALL therapy, were in hematologic complete remission (defined as < 5% blasts in bone marrow, absolute neutrophil count > 1 Gi/L, platelets > 100 Gi/L) and had MRD at a level of ≥ 0.1% using an assay with a minimum sensitivity of 0.01%. BLINCYTO was administered at a constant dose of 15 mcg/m2/day (equivalent to the recommended dosage of 28 mcg/day) intravenously for all treatment cycles. Patients received up to 4 cycles of treatment. Dose adjustment was possible in case of adverse events.
The treated population included 86 patients in first or second hematologic complete remission (CR1 or CR2). The demographics and baseline characteristics are shown in Table 11. The median number of treatment cycles was 2 (range: 1 to 4). Following treatment with BLINCYTO, 45 out of 61 (73.8%) patients in CR1 and 14 out of 25 (56.0%) patients in CR2 underwent allogeneic hematopoietic stem cell transplantation in continuous hematologic complete remission.
| Characteristics | BLINCYTO (N = 86) |
|---|---|
|
|
| Age | |
| Median, years (min, max) | 43 (18, 76) |
| ≥ 65 years, n (%) | 10 (12) |
| Males, n (%) | 50 (58) |
| Race, n (%) | |
| Asian | 1 (1) |
| Other (mixed) | 0 (0) |
| White | 76 (88) |
| Unknown | 9 (11) |
| Philadelphia chromosome disease status, n (%) | |
| Positive | 1 (1) |
| Negative | 85 (99) |
| Relapse history, n (%) | |
| Patients in 1st CR | 61 (71) |
| Patients in 2nd CR | 25 (29) |
| MRD level at baseline*, n (%) | |
| ≥ 10% | 7 (8) |
| ≥ 1% and < 10% | 34 (40) |
| ≥ 0.1% and < 1% | 45 (52) |
Efficacy was based on achievement of undetectable MRD within one cycle of BLINCYTO treatment and hematological relapse-free survival (RFS). The assay used to assess MRD response had a sensitivity of 0.01% for 6 patients and ≤ 0.005% for 80 patients. Overall, undetectable MRD was achieved by 70 patients (81.4%: 95% CI: 71.6%, 89.0%). The median hematological RFS was 22.3 months. Table 12 shows the MRD response and hematological RFS by remission number.
| Patients in CR1 (n = 61) | Patients in CR2 (n = 25) |
|
|---|---|---|
|
||
| Complete MRD response*, n (%), [95% CI] | 52 (85.2) [73.8, 93.0] | 18 (72.0) [50.6, 87.9] |
| Median hematological relapse-free survival† in months (range) | 35.2 (0.4, 53.5) | 12.3 (0.7, 42.3) |
Undetectable MRD was achieved by 65 of 80 patients (81.3%: 95% CI: 71.0%, 89.1%) with an assay sensitivity of at least 0.005%. The estimated median hematological RFS among the 80 patients using the higher sensitivity assay was 24.2 months (95% CI: 17.9, NE).
14.2 Relapsed/Refractory B-cell Precursor ALL
TOWER Study
The efficacy of BLINCYTO was compared to standard of care (SOC) chemotherapy in a randomized, open-label, multicenter study (TOWER Study) [NCT02013167]. Eligible patients were ≥ 18 years of age with relapsed or refractory B-cell precursor ALL [> 5% blasts in the bone marrow and refractory to primary induction therapy or refractory to last therapy, untreated first relapse with first remission duration < 12 months, untreated second or later relapse, or relapse at any time after allogeneic hematopoietic stem cell transplantation (alloHSCT)]. BLINCYTO was administered at 9 mcg/day on Days 1-7 and 28 mcg/day on Days 8-28 for Cycle 1, and 28 mcg/day on Days 1-28 for Cycles 2-5 in 42-day cycles and for Cycles 6-9 in 84-day cycles. Dose adjustment was possible in case of adverse events. SOC chemotherapy included fludarabine, cytarabine arabinoside, and granulocyte colony-stimulating factor (FLAG); high-dose cytarabine arabinoside (HiDAC); high-dose methotrexate- (HDMTX) based combination; or clofarabine/clofarabine-based regimens.
There were 405 patients randomized 2:1 to receive BLINCYTO or investigator-selected SOC chemotherapy. Randomization was stratified by age (< 35 years vs. ≥ 35 years of age), prior salvage therapy (yes vs. no), and prior alloHSCT (yes vs. no) as assessed at the time of consent. The demographics and baseline characteristics were well-balanced between the two arms (see Table 13).
| Characteristics | BLINCYTO (N = 271) | Standard of Care (SOC) Chemotherapy (N = 134) |
|---|---|---|
|
||
| Age | ||
| Median, years (min, max) | 37 (18, 80) | 37 (18, 78) |
| < 35 years, n (%) | 124 (46) | 60 (45) |
| ≥ 35 years, n (%) | 147 (54) | 74 (55) |
| ≥ 65 years, n (%) | 33 (12) | 15 (11) |
| ≥ 75 years, n (%) | 10 (4) | 2 (2) |
| Males, n (%) | 162 (60) | 77 (58) |
| Race, n (%) | ||
| American Indian or Alaska Native | 4 (2) | 1 (1) |
| Asian | 19 (7) | 9 (7) |
| Black (or African American) | 5 (2) | 3 (2) |
| Multiple | 2 (1) | 0 |
| Native Hawaiian or Other Pacific Islander | 1 (0) | 1 (1) |
| Other | 12 (4) | 8 (6) |
| White | 228 (84) | 112 (84) |
| Prior salvage therapy | 171 (63) | 70 (52) |
| Prior alloHSCT* | 94 (35) | 46 (34) |
| Eastern Cooperative Group Status - n (%) | ||
| 0 | 96 (35) | 52 (39) |
| 1 | 134 (49) | 61 (46) |
| 2 | 41 (15) | 20 (15) |
| Unknown | 0 | 1 (1) |
| Refractory to salvage treatment - n (%) | ||
| Yes | 87 (32) | 34 (25) |
| No | 182 (67) | 99 (74) |
| Unknown | 2 (1) | 1 (1) |
| Maximum of central/local bone marrow blasts - n (%) | ||
| ≤ 5% | 0 | 0 |
| > 5 to < 10% | 9 (3) | 7 (5) |
| 10 to < 50% | 60 (22) | 23 (17) |
| ≥ 50% | 201 (74) | 104 (78) |
| Unknown | 1 (0) | 0 |
Of the 271 patients randomized to the BLINCYTO arm, 267 patients received BLINCYTO treatment. The median number of treatment cycles was two (range: 1 to 9 cycles); 267 (99%) received Cycles 1-2 (induction), 86 (32%) received Cycles 3-5 (consolidation), and 27 (10%) received Cycles 6-9 (continued therapy). Of the 134 patients on the SOC arm, 25 dropped out prior to start of study treatment, and 109 patients received a median of 1 treatment cycle (range: 1 to 4 cycles).
The determination of efficacy was based on overall survival (OS). The study demonstrated statistically significant improvement in OS for patients treated with BLINCYTO as compared to SOC chemotherapy.
See Figure 1 and Table 14 below for efficacy results from the TOWER Study.
| Figure 1. Kaplan-Meier Curve of Overall Survival in TOWER Study |
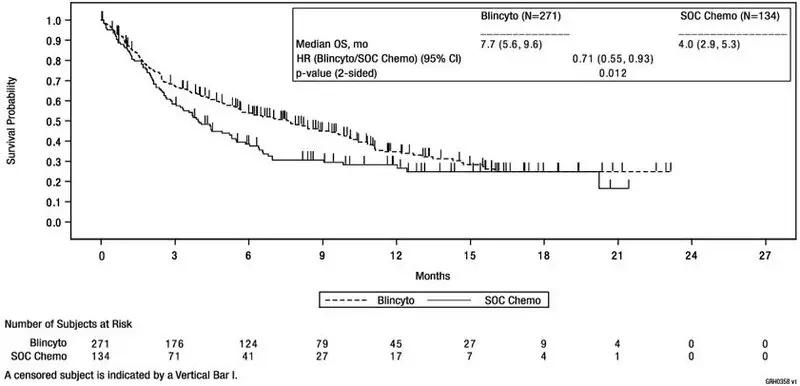 |
| BLINCYTO (N = 271) | SOC Chemotherapy (N = 134) |
|
|---|---|---|
|
||
| Overall Survival | ||
| Number of deaths (%) | 164 (61) | 87 (65) |
| Median, months [95% CI] | 7.7 [5.6, 9.6] | 4.0 [2.9, 5.3] |
| Hazard Ratio [95% CI]* | 0.71 [0.55, 0.93] | |
| p-value† | 0.012 | |
| Overall Response | ||
| CR‡/CRh*§, n (%) [95% CI] | 115 (42) [37, 49] | 27 (20) [14, 28] |
| Treatment difference [95% CI] | 22 [13, 31] | |
| p-value¶ | < 0.001 | |
| CR, n (%) [95% CI] | 91 (34) [28, 40] | 21 (16) [10, 23] |
| Treatment difference [95% CI] | 18 [10, 26] | |
| p-value¶ | < 0.001 | |
| MRD Response# for CR/CRh* | ||
| n1/n2 (%)Þ [95% CI] | 73/115 (64) [54, 72] | 14/27 (52) [32, 71] |
Study MT103-211
Study MT103-211 [NCT01466179] was an open-label, multicenter, single-arm study. Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative relapsed or refractory B-cell precursor ALL (relapsed with first remission duration of ≤ 12 months in first salvage or relapsed or refractory after first salvage therapy or relapsed within 12 months of alloHSCT, and had ≥ 10% blasts in bone marrow).
BLINCYTO was administered as a continuous intravenous infusion. The recommended dose for this study was determined to be 9 mcg/day on Days 1-7 and 28 mcg/day on Days 8-28 for Cycle 1, and 28 mcg/day on Days 1-28 for subsequent cycles. Dose adjustment was possible in case of adverse events. The treated population included 185 patients who received at least 1 infusion of BLINCYTO; the median number of treatment cycles was 2 (range: 1 to 5). Patients who responded to BLINCYTO but later relapsed had the option to be retreated with BLINCYTO. Among treated patients, the median age was 39 years (range: 18 to 79 years), 63 out of 185 (34.1%) had undergone HSCT prior to receiving BLINCYTO, and 32 out of 185 (17.3%) had received more than 2 prior salvage therapies.
Efficacy was based on the complete remission (CR) rate, duration of CR, and proportion of patients with an MRD-negative CR/CR with partial hematological recovery (CR/CRh*) within 2 cycles of treatment with BLINCYTO. Table 15 shows the efficacy results from this study. The HSCT rate among those who achieved CR/CRh* was 39% (30 out of 77).
| N = 185 | |||
|---|---|---|---|
| CR* | CRh*† | CR/CRh* | |
|
|||
| n (%) [95% CI] | 60 (32.4) [25.7, 39.7] | 17 (9.2) [5.4, 14.3] | 77 (41.6) [34.4, 49.1] |
| MRD response‡ | |||
| n1/n2 (%)§
[95% CI] | 48/60 (80.0) [67.7, 89.2] | 10/17 (58.8) [32.9, 81.6] | 58/77 (75.3) [64.2, 84.4] |
| DOR/RFS¶ | |||
| Median (months) (range) | 6.7 (0.46 – 16.5) | 5.0 (0.13 – 8.8) | 5.9 (0.13 – 16.5) |
ALCANTARA Study
The efficacy of BLINCYTO for treatment of Philadelphia chromosome-positive B-cell precursor ALL was evaluated in an open-label, multicenter, single-arm study (ALCANTARA Study) [NCT02000427]. Eligible patients were ≥ 18 years of age with Philadelphia chromosome-positive B-cell precursor ALL, relapsed or refractory to at least 1 second generation or later tyrosine kinase inhibitor (TKI), or intolerant to second generation TKI, and intolerant or refractory to imatinib mesylate.
BLINCYTO was administered at 9 mcg/day on Days 1-7 and 28 mcg/day on Days 8-28 for Cycle 1, and 28 mcg/day on Days 1-28 for subsequent cycles. Dose adjustment was possible in case of adverse events.
The treated population included 45 patients who received at least one infusion of BLINCYTO; the median number of treatment cycles was 2 (range: 1 to 5). The demographics and baseline characteristics are shown in Table 16.
| Characteristics | BLINCYTO (N = 45) |
|---|---|
|
|
| Age | |
| Median, years (min, max) | 55 (23, 78) |
| ≥ 65 years and < 75 years, n (%) | 10 (22) |
| ≥ 75 years, n (%) | 2 (4) |
| Males, n (%) | 24 (53) |
| Race, n (%) | |
| Asian | 1 (2) |
| Black (or African American) | 3 (7) |
| Other | 2 (4) |
| White | 39 (87) |
| Disease History | |
| Prior TKI treatment*, n (%) | |
| 1 | 7 (16) |
| 2 | 21 (47) |
| ≥ 3 | 17 (38) |
| Prior salvage therapy | 31 (62) |
| Prior alloHSCT† | 20 (44) |
| Bone marrow blasts‡ | |
| ≥ 50% to < 75% | 6 (13) |
| ≥ 75% | 28 (62) |
Efficacy was based on the complete remission (CR) rate, duration of CR, and proportion of patients with an MRD-negative CR/CR with partial hematological recovery (CR/CRh*) within 2 cycles of treatment with BLINCYTO. Table 17 shows the efficacy results from ALCANTARA Study. Five of the 16 responding (31%) patients underwent allogeneic HSCT in CR/CRh* induced with BLINCYTO. There were 10 patients with documented T315I mutation; four achieved CR within 2 cycles of treatment with BLINCYTO.
| N = 45 | |||
|---|---|---|---|
| CR* | CRh*† | CR/CRh* | |
|
|||
| n (%) [95% CI] | 14 (31) [18, 47] | 2 (4) [1, 15] | 16 (36) [22, 51] |
| MRD response‡ | |||
| n1/n2 (%)§
[95% CI] | 12/14 (86) [57, 98] | 2/2 (100) [16, 100] | 14/16 (88) [62, 98] |
| DOR/RFS¶ | |||
| Median (months) (range) | 6.7 (3.6 – 12.0) | NE# (3.7 – 9.0) | 6.7 (3.6 – 12.0) |
Study MT103-205
Study MT103-205 [NCT01471782] was an open-label, multicenter, single-arm study in pediatric patients with relapsed or refractory B-cell precursor ALL (second or later bone marrow relapse, any marrow relapse after allogeneic HSCT, or refractory to other treatments, and had > 25% blasts in bone marrow). BLINCYTO was administered at 5 mcg/m2/day on Days 1-7 and 15 mcg/m2/day on Days 8-28 for Cycle 1, and 15 mcg/m2/day on Days 1-28 for subsequent cycles. Dose adjustment was possible in case of adverse events. Patients who responded to BLINCYTO but later relapsed had the option to be retreated with BLINCYTO.
Among the 70 treated patients, the median age was 8 years (range: 7 months to 17 years), 40 out of 70 (57.1%) had undergone allogeneic HSCT prior to receiving BLINCYTO, and 39 out of 70 (55.7%) had refractory disease. The median number of treatment cycles was 1 (range: 1 to 5).
Twenty-three out of 70 (32.9%) patients achieved CR/CRh* within the first 2 treatment cycles with 17 out of 23 (73.9%) occurring within Cycle 1 of treatment. See Table 18 for the efficacy results from the study. The HSCT rate among those who achieved CR/CRh* was 48% (11 out of 23).
| N = 70 | |||
|---|---|---|---|
| CR* | CRh*† | CR/CRh* | |
|
|||
| n (%) [95% CI] | 12 (17.1) [9.2, 28.0] | 11 (15.7) [8.1, 26.4] | 23 (32.9) [22.1, 45.1] |
| MRD response‡ | |||
| n1/n2 (%)§
[95% CI] | 6/12 (50.0) [21.1, 78.9] | 4/11 (36.4) [10.9, 69.2] | 10/23 (43.5) [23.2, 65.5] |
| DOR/RFS¶ | |||
| Median (months) (range) | 6.0 (0.5 – 12.1) | 3.5 (0.5 – 16.4) | 6.0 (0.5 – 16.4) |
16. How is Blincyto supplied
Each BLINCYTO package (NDC 55513-160-01) contains:
- One BLINCYTO (blinatumomab) for injection 35 mcg single-dose vial containing a sterile, preservative-free, white to off-white lyophilized powder and
- One IV Solution Stabilizer 10 mL single-dose glass vial containing a sterile, preservative-free, colorless to slightly yellow, clear solution.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 6/2023 | ||
| Medication Guide
BLINCYTO® (blin sye toe) (blinatumomab) for injection |
|||
| What is the most important information I should know about BLINCYTO?
Call your healthcare provider or get emergency medical help right away if you get any of the symptoms listed below. BLINCYTO may cause serious side effects that can be severe, life-threatening, or lead to death, including: |
|||
|
|||
|
|
||
|
|||
|
|
||
| Your healthcare provider will check for these problems during treatment with BLINCYTO. Your healthcare provider may temporarily stop or completely stop your treatment with BLINCYTO, if you have severe side effects. See “What are the possible side effects of BLINCYTO?” below for other side effects of BLINCYTO. |
|||
| What is BLINCYTO?
BLINCYTO is a prescription medicine used to treat adults and children with:
|
|||
| Who should not receive BLINCYTO?
Do not receive BLINCYTO if you are allergic to blinatumomab or to any of the ingredients of BLINCYTO. See the end of this Medication Guide for a complete list of ingredients in BLINCYTO. |
|||
Before receiving BLINCYTO, tell your healthcare provider about all of your medical conditions, including if you or your child:
|
|||
How will I receive BLINCYTO?
|
|||
| What should I avoid while receiving BLINCYTO?
Do not drive, operate heavy machinery, or do other dangerous activities while you are receiving BLINCYTO because BLINCYTO can cause neurological symptoms, such as dizziness, seizures, and confusion. |
|||
| What are the possible side effects of BLINCYTO?
BLINCYTO may cause serious side effects, including: See “What is the most important information I should know about BLINCYTO?”
|
|||
|
|
||
|
|||
|
|
||
| These are not all the possible side effects of BLINCYTO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
| How should I store BLINCYTO?
Intravenous (IV) bags containing BLINCYTO for infusion will arrive in a special package.
|
|||
| General information about safe and effective use of BLINCYTO
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BLINCYTO for a condition for which it was not prescribed. Do not give BLINCYTO to other people even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BLINCYTO that is written for health professionals. |
|||
| What are the ingredients in BLINCYTO?
Active ingredient: blinatumomab Inactive ingredients: citric acid monohydrate, lysine hydrochloride, polysorbate 80, trehalose dihydrate, sodium hydroxide and preservative-free sterile water for injection. Inactive ingredients of IV Solution Stabilizer: citric acid monohydrate, lysine hydrochloride, polysorbate 80, sodium hydroxide and water for injection. Manufactured by: Amgen Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799 U.S. License No. 1080 © 2014-2022 Amgen Inc. All rights reserved. V9 For more information, go to www.blincyto.com or call Amgen at 1-800-772-6436. |
|||
| BLINCYTO
blinatumomab kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Amgen Inc (039976196) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins PROXY Laboratories B.V. | 490477955 | ANALYSIS(55513-160) | |





