Drug Detail:Botox cosmetic (Onabotulinumtoxina (botox) [ on-a-bot-ue-lye-num-tox-in-a ])
Drug Class: Skeletal muscle relaxants
Highlights of Prescribing Information
BOTOX Cosmetic (onabotulinumtoxinA) for injection,
for intramuscular use
Initial U.S. Approval: 1989
WARNING: DISTANT SPREAD OF TOXIN EFFECT
See full prescribing information for complete boxed warning.
The effects of BOTOX Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms. (5.2)
Recent Major Changes
- Warnings and Precautions, Dry Eye in Patients Treated with BOTOX Cosmetic (5.10) 11/2019
Indications and Usage for Botox Cosmetic
BOTOX Cosmetic is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated in adult patients for the temporary improvement in the appearance of (1):
- Moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
- Moderate to severe lateral canthal lines associated with orbicularis oculi activity
- Moderate to severe forehead lines associated with frontalis muscle activity
Botox Cosmetic Dosage and Administration
- Botox Cosmetic is administered by intramuscular injection
- Glabellar Lines Administration: 0.1 mL (4 Units) into each of 5 sites, for a total dose of 20 Units (2.3)
- Lateral Canthal Lines Administration: 0.1 mL (4 Units) into each of 3 sites per side (6 total injection points), for a total of 24 Units (2.3)
- Forehead Lines Administration: 0.1 mL (4 Units) into each of 5 forehead line sites (20 Units) with 0.1 mL (4 Units) into each of 5 glabellar line sites (20 Units), for a recommended total of 40 Units (2.3)
- Follow dosage and administration recommendations. In treating adults for more than one approved indications with BOTOX and BOTOX Cosmetic, do not exceed a total dose of 400 Units administered in a 3 month interval (2.1)
- See Preparation and Dilution Technique for instructions on BOTOX Cosmetic reconstitution, storage, and preparation before injection (2.2)
Dosage Forms and Strengths
For Injection: 50 Units or 100 Units vacuum-dried powder in a single-dose vial for reconstitution (3)
Contraindications
- Hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation (4.1, 5.4)
- Infection at the injection site (4.2)
Warnings and Precautions
- Potency Units of BOTOX Cosmetic are not interchangeable with other preparations of botulinum toxin products (5.1, 11)
- Spread of toxin effects; swallowing and breathing difficulties can lead to death. Seek immediate medical attention if respiratory, speech or swallowing difficulties occur (5.2, 5.7)
- Potential serious adverse reactions after administration of BOTOX for unapproved uses (5.3)
- Adverse event reports have been received involving the cardiovascular system, some with fatal outcomes. Use caution when administering to patients with pre-existing cardiovascular disease. (5.5)
- Concomitant neuromuscular disorder may exacerbate clinical effects of treatment (5.6)
- Use with caution in patients with compromised respiratory function or dysphagia (5.7)
Adverse Reactions/Side Effects
The most common adverse reactions are (6.1):
- Glabellar Lines: eyelid ptosis (3%)
- Lateral Canthal Lines: eyelid edema (1%)
- Forehead Lines: headache (9%) and brow ptosis (2%)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Patients receiving concomitant treatment of BOTOX Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents), or muscle relaxants, should be observed closely because the effect of BOTOX Cosmetic may be potentiated (7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2020
Full Prescribing Information
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses. [see Warnings and Precautions (5.2)]
1. Indications and Usage for Botox Cosmetic
BOTOX Cosmetic (onabotulinumtoxinA) is indicated in adult patients for the temporary improvement in the appearance of:
- moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
- moderate to severe lateral canthal lines associated with orbicularis oculi activity
- moderate to severe forehead lines associated with frontalis muscle activity
2. Botox Cosmetic Dosage and Administration
2.1 Instructions for Safe Use
The potency Units of BOTOX Cosmetic (onabotulinumtoxinA) for injection are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method [see Warnings and Precautions (5.1) and Description (11)].
Indication specific dosage and administration recommendations should be followed. In treating adult patients for one or more indications with BOTOX and BOTOX Cosmetic, the maximum cumulative dose should generally not exceed 400 Units, in a 3 month interval.
The safety and effectiveness of dosing with BOTOX Cosmetic more frequently than every 3 months have not been clinically evaluated.
The safe and effective use of BOTOX Cosmetic depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques. Physicians administering BOTOX Cosmetic must understand the relevant neuromuscular and structural anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures and disease.
Do not use BOTOX Cosmetic and contact Allergan (1-800-890-4345) if:
- The carton labeling does not contain an intact seal with a translucent silver Allergan logo (on both ends of the carton) or the seal has a black circle with a diagonal line through it (i.e., prohibition sign)
- The vial label does not contain a holographic film containing the name “Allergan” within rainbow-colored horizontal lines
- The U.S. License number 1145 is not present on the vial label and carton labeling [see How Supplied/Storage and Handling (16)]
2.2 Preparation and Dilution Technique
BOTOX Cosmetic is supplied in single-dose 50 Units and 100 Units per vial. Prior to intramuscular injection, reconstitute each vacuum-dried vial of BOTOX Cosmetic with sterile, preservative-free 0.9% Sodium Chloride Injection USP (see Table 1). Draw up the proper amount of diluent in the appropriate size needle and syringe to obtain a reconstituted solution at a concentration of 4 Units/0.1 mL and a total treatment dose of 20 Units in 0.5 mL for glabellar lines, 24 Units in 0.6 mL for lateral canthal lines, and 40 Units in 1 mL for forehead lines and glabellar lines. Then slowly inject the diluent into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Gently mix BOTOX Cosmetic with the saline by rotating the vial. Record the date and time of reconstitution on the space on the label. BOTOX Cosmetic should be administered within 24 hours after reconstitution. During this time period, reconstituted BOTOX Cosmetic should be stored in a refrigerator (2° to 8°C). BOTOX Cosmetic vials are for single-dose only. Discard any remaining solution.
| Diluent* Added to
100 Unit Vial | Resulting Dose Units per 0.1 mL | Diluent* Added to
50 Unit Vial | Resulting Dose Units per 0.1 mL |
| 2.5 mL | 4 Units | 1.25 mL | 4 Units |
*Preservative-free 0.9% Sodium Chloride Injection, USP Only
Reconstituted BOTOX Cosmetic should be clear, colorless, and free of particulate matter. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and whenever the solution and the container permit. Do not freeze reconstituted BOTOX Cosmetic.
2.3 Administration
Draw at least 0.5 mL (for glabellar lines), 0.6 mL (for lateral canthal lines), or 1 mL (for forehead lines treated in conjunction with glabellar lines) of the properly reconstituted toxin into the sterile syringe, preferably a tuberculin syringe and expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach a 30-33 gauge needle. Confirm the patency of the needle.
Glabellar Lines
Glabellar facial lines arise from the activity of the corrugator and orbicularis oculi muscles. These muscles move the brow medially, and the procerus and depressor supercilii pull the brow inferiorly. This creates a frown or “furrowed brow”. The location, size, and use of the muscles vary markedly among individuals. Lines induced by facial expression occur perpendicular to the direction of action of contracting facial muscles. An effective dose for facial lines is determined by gross observation of the patient’s ability to activate the superficial muscles injected.
In order to reduce the complication of ptosis the following steps should be taken:
- Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
- Lateral corrugator injections should be placed at least 1 cm above the bony supraorbital ridge.
- Ensure the injected volume/dose is accurate and where feasible kept to a minimum.
- Do not inject toxin closer than 1 cm above the central eyebrow.
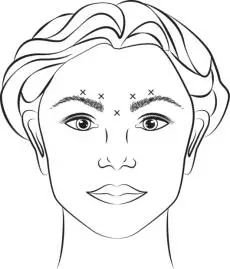
Inject 4 Units (0.1 mL) of reconstituted BOTOX Cosmetic intramuscularly into each of 5 sites, 2 in each corrugator muscle and 1 in the procerus muscle for a total dose of 20 Units (see Figure 1). Typically the initial doses of reconstituted BOTOX Cosmetic induce chemical denervation of the injected muscles one to two days after injection, increasing in intensity during the first week.
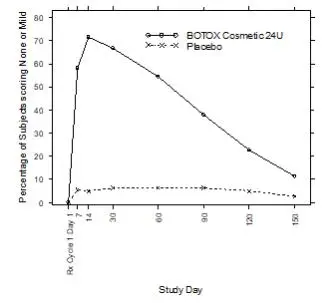
The duration of effect of BOTOX Cosmetic for glabellar lines is approximately 3-4 months.
Figure 1:
Lateral Canthal Lines
Lateral canthal lines arise largely from the activity of the orbicularis oculi muscles around the eye responsible for blinking and eyelid closure. Forceful contraction of the orbicularis oculi results in lateral and radially oriented folds (crow’s feet lines) which originate from the lateral canthus. The distribution of these radial lines differs among patients.
Injections should be given with the needle bevel tip up and oriented away from the eye. Inject 4 Units/0.1 mL of reconstituted BOTOX Cosmetic into 3 sites per side (6 total injection points) in the lateral orbicularis oculi muscle for a total of 24 Units/0.6 mL (12 Units per side). The first injection (A) should be approximately 1.5-2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. If the lines in the lateral canthal region are above and below the lateral canthus, inject per Figure 2. Alternatively, if the lines in the lateral canthal region are primarily below the lateral canthus, inject per Figure 3.
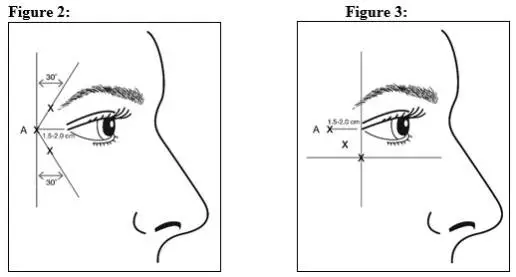
For simultaneous treatment with glabellar lines, the dose is 24 Units for lateral canthal lines and 20 Units for glabellar lines (see Glabellar Lines Administration and Figure 1), with a total dose of 44 Units.
Forehead Lines
Forehead lines arise largely from the activity of the frontalis muscles. This muscle moves the brow superiorly, interacting with the procerus, orbicularis, corrugator, and depressor supercilli. Frontalis contraction causes brow elevation. The location, size, and use of the muscles vary markedly among individuals.
Treat forehead lines in conjunction with glabellar lines (see Glabellar Lines Administration and Figure 1) to minimize the potential for brow ptosis. The recommended total dose for treatment of forehead lines (20 Units [0.5 mL]) in conjunction with glabellar lines (20 Units [0.5 mL]) is 40 Units (1mL).
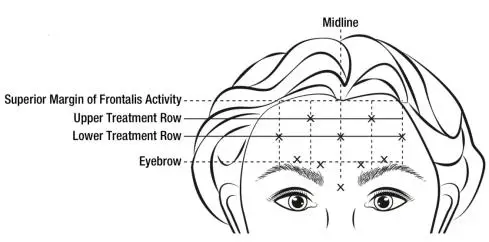
When identifying the location of the appropriate injection sites in the frontalis muscle, assess the overall relationship between the size of the subject’s forehead, and the distribution of frontalis muscle activity.
Locate the following horizontal treatment rows by light palpation of the forehead at rest and maximum eyebrow elevation:
- Superior Margin of Frontalis Activity: approximately 1 cm above the most superior forehead crease
- Lower Treatment Row: midway between the superior margin of frontalis activity and the eyebrow, at least 2 cm above the eyebrow
- Upper Treatment Row: midway between the superior margin of frontalis activity and lower treatment row
Inject 4 Units (0.1 mL) of reconstituted BOTOX Cosmetic into 5 sites in the frontalis muscle, for a total of 20 Units (0.5 mL). Place the 5 injections at the intersection of the horizontal treatment rows with the following vertical landmarks (see Figure 4):
- On the lower treatment row at the midline of the face, and 0.5 – 1.5 cm medial to the palpated temporal fusion line (temporal crest); repeat for the other side.
- On the upper treatment row, midway between the lateral and medial sites on the lower treatment row; repeat for the other side.
Figure 4:
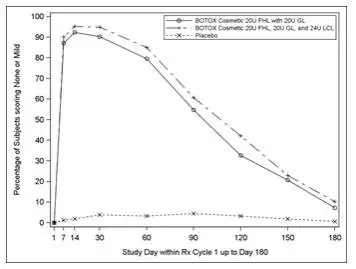
For simultaneous treatment with lateral canthal lines, the total dose is 64 Units, comprised of 20 Units for forehead lines, 20 Units for glabellar lines, and 24 Units for lateral canthal lines (see Lateral Canthal Lines Administration and Figures 2 and 3).
3. Dosage Forms and Strengths
- For injection: 50 Units, vacuum-dried powder in a single-dose vial for reconstitution
- For injection: 100 Units, vacuum-dried powder in a single-dose vial for reconstitution
4. Contraindications
5. Warnings and Precautions
5.1 Lack of Interchangeability between Botulinum Toxin Products
The potency Units of BOTOX Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method [see Description (11)].
5.2 Spread of Toxin Effect
Postmarketing safety data from BOTOX Cosmetic and other approved botulinum toxins suggest that botulinum toxin effects may, in some cases, be observed beyond the site of local injection. The symptoms are consistent with the mechanism of action of botulinum toxin and may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death related to spread of toxin effects. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, and particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses and in approved indications, symptoms consistent with spread of toxin effect have been reported at doses comparable to or lower than doses used to treat cervical dystonia and spasticity. Patients or caregivers should be advised to seek immediate medical care if swallowing, speech or respiratory disorders occur.
No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX/BOTOX Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines), or 100 Units (for severe primary axillary hyperhidrosis) have been reported.
No definitive serious adverse event reports of distant spread of toxin effect associated with BOTOX for blepharospasm at the recommended dose (30 Units and below), strabismus, or chronic migraine at the labeled doses have been reported.
5.3 Serious Adverse Reactions with Unapproved Use
Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, with some adverse reactions associated with fatal outcomes, have been reported in patients who received BOTOX injections for unapproved uses. In these cases, the adverse reactions were not necessarily related to distant spread of toxin, but may have resulted from the administration of BOTOX to the site of injection and/or adjacent structures. In several of the cases, patients had pre-existing dysphagia or other significant disabilities. There is insufficient information to identify factors associated with an increased risk for adverse reactions associated with the unapproved uses of BOTOX. The safety and effectiveness of BOTOX for unapproved uses have not been established.
5.4 Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea. If such a reaction occurs, further injection of BOTOX Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent, and consequently the causal agent cannot be reliably determined.
5.5 Cardiovascular System
There have been reports following administration of BOTOX/BOTOX Cosmetic of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
5.6 Increased Risk of Clinically Significant Effects with Pre-Existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including generalized muscle weakness, diplopia, ptosis, dysphonia, dysarthria, severe dysphagia and respiratory compromise from onabotulinumtoxinA [see Warnings and Precautions (5.2, 5.7)].
5.7 Dysphagia and Breathing Difficulties
Treatment with BOTOX and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or oropharyngeal muscles that control swallowing or breathing [see Warnings and Precautions (5.2)].
Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several months, and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised.
Treatment with botulinum toxins may weaken neck muscles that serve as accessory muscles of ventilation. This may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these accessory muscles. There have been postmarketing reports of serious breathing difficulties, including respiratory failure.
Patients with smaller neck muscle mass and patients who require bilateral injections into the sternocleidomastoid muscle for the treatment of cervical dystonia have been reported to be at greater risk for dysphagia. Limiting the dose injected into the sternocleidomastoid muscle may reduce the occurrence of dysphagia. Injections into the levator scapulae may be associated with an increased risk of upper respiratory infection and dysphagia.
Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin [see Warnings and Precautions (5.2)].
5.9 Corneal Exposure and Ulceration in Patients Treated with BOTOX for Blepharospasm
Reduced blinking from injection of botulinum toxin products in or near the orbicularis oculi muscle can lead to corneal exposure, persistent corneal epithelial defect, and corneal ulceration, especially in patients with VII nerve disorders.
Vigorous treatment of any corneal epithelial defect should be employed. This may require protective drops, ointment, therapeutic soft contact lenses, or closure of the eye by patching or other means.
5.10 Dry Eye in Patients Treated with BOTOX Cosmetic
There have been reports of dry eye associated with BOTOX Cosmetic injection in or near the orbicularis oculi muscle. If symptoms of dry eye (e.g., eye irritation, photophobia, or visual changes) persist, consider referring patients to an ophthalmologist [see Warnings and Precautions (5.9)].
6. Adverse Reactions/Side Effects
The following adverse reactions to BOTOX Cosmetic (onabotulinumtoxinA) for injection are discussed in greater detail in other sections of the labeling:
- Spread of Toxin Effects [see Warnings and Precautions (5.2)]
- Hypersensitivity [see Contraindications (4.1) and Warnings and Precautions (5.4)]
- Dysphagia and Breathing Difficulties [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
BOTOX and BOTOX Cosmetic contain the same active ingredient in the same formulation, but have different labeled Indications and Usage. Therefore, adverse events observed with the use of BOTOX also have the potential to be observed with the use of BOTOX Cosmetic.
In general, adverse reactions occur within the first week following injection of BOTOX Cosmetic and while generally transient, may have a duration of several months or longer. Localized pain, infection, inflammation, tenderness, swelling, erythema, and/or bleeding/bruising may be associated with the injection. Needle-related pain and/or anxiety may result in vasovagal responses (including e.g., syncope, hypotension), which may require appropriate medical therapy.
Local weakness of the injected muscle(s) represents the expected pharmacological action of botulinum toxin. However, weakness of nearby muscles may also occur due to spread of toxin [see Warnings and Precautions (5.2)].
Glabellar Lines
Table 2 lists selected adverse reactions reported by ≥1% of BOTOX Cosmetic treated subjects (N=405) aged 18 to 75 who were evaluated in the randomized, placebo-controlled clinical studies to assess the use of BOTOX Cosmetic in the improvement of the appearance of glabellar lines.
| Adverse Reactions by System Organ Class | BOTOX Cosmetic
(N=405) | Placebo
(N=130) |
| General Disorders and Administration Site Conditions Facial pain | 6 (1%) | 0 (0%) |
| Nervous System Disorders Facial paresis | 5 (1%) | 0 (0%) |
| Eye Disorders Eyelid ptosis | 13 (3%) | 0 (0%) |
| Musculoskeletal and Connective Tissue Disorders Muscular Weakness | 6 (1%) | 0 (0%) |
Lateral Canthal Lines
Table 3 lists selected adverse reactions reported within 90 days following injection by >1% of BOTOX Cosmetic treated subjects (N=526) aged 18 to 75 who were evaluated in two randomized, double-blind, placebo-controlled clinical studies to assess the use of BOTOX Cosmetic in the improvement of the appearance of lateral canthal lines alone.
| Adverse Reactions by System Organ Class | BOTOX Cosmetic 24 Units
(N=526) | Placebo
(N=530) |
| Eye disorders Eyelid edema |
5 (1%) |
0 (0%) |
Forehead Lines
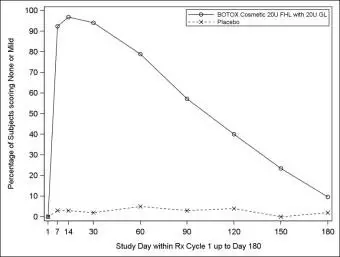
Table 4 lists selected adverse reactions reported by >1% of BOTOX Cosmetic treated subjects (N=665) aged 18 to 77 who were evaluated in two randomized, double-blind, placebo-controlled clinical studies to assess the use of BOTOX Cosmetic in the improvement of the appearance of forehead lines with glabellar lines.
| Adverse Reactions by System Organ Class | BOTOX Cosmetic
(20 Units forehead lines with 20 Units glabellar lines) (N=665) | Placebo
(N=315) |
| Nervous System Disorders Headache | 58 (9%) | 17 (5%) |
| Eye Disorders Eyelid ptosis | 12 (2%) | 1 (0%) |
| Skin and Subcutaneous Tissue Disorders Brow ptosis Skin tightness | 13 (2%) 10 (2%) | 0 (0%) 0 (0%) |
There were no additional adverse drug reactions reported with the simultaneous treatment of forehead lines, glabellar lines, and lateral canthal lines.
7. Drug Interactions
No formal drug interaction studies have been conducted with BOTOX Cosmetic (onabotulinumtoxinA) for injection.
11. Botox Cosmetic Description
BOTOX Cosmetic (onabotulinumtoxinA) for injection, is a sterile, vacuum-dried purified botulinum toxin type A, produced from fermentation of Hall strain Clostridium botulinum type A intended for intramuscular use. It is purified from the culture solution by dialysis and a series of acid precipitations to a complex consisting of the neurotoxin, and several accessory proteins. The complex is dissolved in sterile sodium chloride solution containing Albumin Human and is sterile filtered (0.2 microns) prior to filling and vacuum-drying.
The primary release procedure for BOTOX Cosmetic uses a cell-based potency assay to determine the potency relative to a reference standard. The assay is specific to Allergan’s products BOTOX and BOTOX Cosmetic. One Unit of BOTOX Cosmetic corresponds to the calculated median intraperitoneal lethal dose (LD50) in mice. Due to specific details of this assay such as the vehicle, dilution scheme and laboratory protocols, Units of biological activity of BOTOX Cosmetic cannot be compared to nor converted into Units of any other botulinum toxin or any toxin assessed with any other specific assay method. The specific activity of BOTOX Cosmetic is approximately 20 Units/nanogram of neurotoxin complex.
Each vial of BOTOX Cosmetic contains either 50 Units of Clostridium botulinum type A neurotoxin complex, 0.25 mg of Albumin Human, and 0.45 mg of sodium chloride; or 100 Units of Clostridium botulinum type A neurotoxin complex, 0.5 mg of Albumin Human, and 0.9 mg of sodium chloride in a sterile, vacuum-dried form without a preservative.
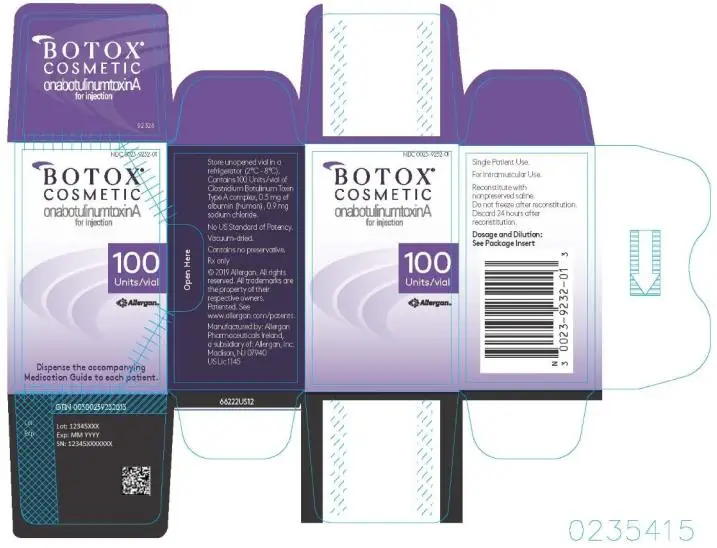
16. How is Botox Cosmetic supplied
BOTOX Cosmetic (onabotulinumtoxinA) for injection is a vacuum-dried powder supplied in a single-dose vial in the following sizes:
50 Units: NDC 0023-3919-50
100 Units: NDC 0023-9232-01
The top and bottom flaps of the BOTOX Cosmetic cartons have a tamper-evident seal that contains a translucent silver Allergan logo, and the BOTOX Cosmetic vial labels have a holographic film that contains the name “Allergan” within rainbow-colored horizontal lines (rotate the vial back and forth between your fingers under a desk lamp or fluorescent light source to see the hologram). (Note: The holographic film on the label is absent in the date/lot area.) Each BOTOX Cosmetic vial label and carton labeling also contain the U.S. License number 1145 [see Dosage and Administration (2.1)].
Do not use the product and contact Allergan for additional information at 1-800-890-4345 from 7:00 AM to 3:00 PM Pacific Time if the labeling is not as described above.
Storage
Unopened vials of BOTOX Cosmetic should be stored in a refrigerator 2° to 8°C (36º to 46ºF). Do not use after the expiration date on the vial. Reconstituted BOTOX Cosmetic should be stored in a refrigerator 2° to 8°C (36º to 46ºF) and administered within 24 hours.
Medication Guide
| MEDICATION GUIDE
BOTOX® BOTOX® Cosmetic (Boe-tox) (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use |
| What is the most important information I should know about BOTOX and BOTOX Cosmetic?
BOTOX and BOTOX Cosmetic may cause serious side effects that can be life threatening, including:
Call your doctor or get medical help right away if you have any of these problems after treatment with BOTOX or BOTOX Cosmetic:
These problems could make it unsafe for you to drive a car or do other dangerous activities. See "What should I avoid while receiving BOTOX or BOTOX Cosmetic?" There has not been a confirmed serious case of spread of toxin effect away from the injection site when BOTOX has been used at the recommended dose to treat chronic migraine, severe underarm sweating, blepharospasm, or strabismus, or when BOTOX Cosmetic has been used at the recommended dose to treat frown lines, crow’s feet lines, and/or forehead lines. |
| What are BOTOX and BOTOX Cosmetic?
BOTOX is a prescription medicine that is injected into muscles and used:
|
BOTOX Cosmetic is a prescription medicine for adults that is injected into muscles and used for a short period of time (temporary) to improve the look of:
|
It is not known whether BOTOX is safe and effective in people younger than:
It is not known whether BOTOX and BOTOX Cosmetic are safe and effective to prevent headaches in people with migraine who have 14 or fewer headache days each month (episodic migraine). It is not known whether BOTOX and BOTOX Cosmetic are safe and effective for severe sweating anywhere other than your armpits. It is not known if BOTOX Cosmetic is safe and effective for use more than 1 time every 3 months. |
| Who should not receive BOTOX or BOTOX Cosmetic?
Do not receive BOTOX or BOTOX Cosmetic if you:
|
| What should I tell my doctor before receiving BOTOX or BOTOX Cosmetic?
Tell your doctor about all your medical conditions, including if you:
Using BOTOX or BOTOX Cosmetic with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received BOTOX or BOTOX Cosmetic in the past. Especially tell your doctor if you:
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine. |
How will I receive BOTOX or BOTOX Cosmetic?
|
| What should I avoid while receiving BOTOX or BOTOX Cosmetic?
BOTOX and BOTOX Cosmetic may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours to weeks of taking BOTOX or BOTOX Cosmetic. If this happens, do not drive a car, operate machinery, or do other dangerous activities. See "What is the most important information I should know about BOTOX and BOTOX Cosmetic?" |
| What are the possible side effects of BOTOX and BOTOX Cosmetic?
BOTOX and BOTOX Cosmetic can cause serious side effects. See "What is the most important information I should know about BOTOX and BOTOX Cosmetic?" Other side effects of BOTOX and BOTOX Cosmetic include:
These are not all the possible side effects of BOTOX and BOTOX Cosmetic. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
| General information about the safe and effective use of BOTOX and BOTOX Cosmetic:
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. This Medication Guide summarizes the most important information about BOTOX and BOTOX Cosmetic. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about BOTOX and BOTOX Cosmetic that is written for health professionals. |
| What are the ingredients in BOTOX and BOTOX Cosmetic?
Active ingredient: onabotulinumtoxinA Inactive ingredients: human albumin and sodium chloride |
| Manufactured by: Allergan Pharmaceuticals Ireland a subsidiary of: Allergan, Inc. U.S. License Number 1145 Distributed by: Allergan USA, Inc. Madison, NJ 07940 ©2023 Allergan. All rights reserved. All trademarks are the property of their respective owners. Patented. See: www.allergan.com/patents  v6.0MG1145 |
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 8/2023
| BOTOX COSMETIC
onabotulinumtoxina injection, powder, lyophilized, for solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| BOTOX COSMETIC
onabotulinumtoxina injection, powder, lyophilized, for solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |