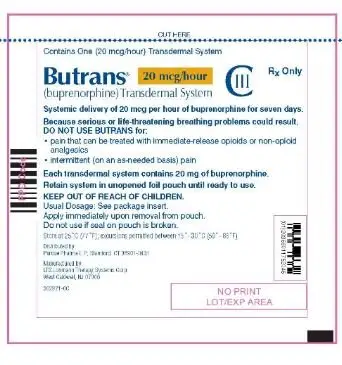
Drug Detail:Butrans skin patch (Buprenorphine transdermal (skin patch) [ bue-pre-nor-feen ])
Drug Class: Opioids (narcotic analgesics)
Highlights of Prescribing Information
BUTRANS® (buprenorphine) transdermal system, CIII
Initial U.S. Approval: 1981
WARNING: ADDICTION, ABUSE, and MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL EXPOSURE; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
See full prescribing information for complete boxed warning.
- BUTRANS exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient’s risk before prescribing, and monitor for these behaviors and conditions. (5.1, 10)
- To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products. (5.2)
- Serious, life-threatening or fatal respiratory depression may occur. Monitor closely, especially upon initiation or following a dose increase. Instruct patients on proper administration of BUTRANS to reduce the risk. (5.3)
- Accidental exposure to BUTRANS, especially in children, can result in fatal overdose of buprenorphine. (5.3)
- Prolonged use of BUTRANS during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. (5.4)
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation. (5.5, 7)
Recent Major Changes
Indications and Usage for Butrans Patch
BUTRANS is a partial opioid agonist indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate. (1)
Limitations of Use
- Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, and because of the greater risks of overdose and death with extended-release opioid formulations, reserve BUTRANS for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or immediate-release opioids) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain. (1)
- BUTRANS is not indicated as an as-needed (prn) analgesic. (1)
Butrans Patch Dosage and Administration
- To be prescribed only by healthcare providers knowledgeable in use of potent opioids for management of chronic pain. (2.1)
- BUTRANS doses of 7.5, 10, 15, and 20 mcg/hour are only for use in patients who are opioid experienced and in whom tolerance to an opioid of comparable potency has been established. Patients who are opioid-experienced are those receiving, for one week or longer, daily opioid doses up to 80 mg/day of oral morphine or an equianalgesic dose of another opioid. (2.1)
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals (2.1).
Individualize dosing based on the severity of pain, patient response, prior analgesic experience, and risk factors for addiction, abuse, and misuse. (2.1)
- For opioid-naïve patients, initiate treatment with a 5 mcg/hour patch. (2.1)
- Instruct patients to wear BUTRANS for 7 days and to wait a minimum of 3 weeks before applying to the same site. (2.1)
- Discuss availability of naloxone with the patient and caregiver and assess each patient’s need for access to naloxone, both when initiating and renewing treatment with BUTRANS. Consider prescribing naloxone based on the patient’s risk factors for overdose. (2.2, 5.1, 5.3, 5.5, 10)
- Do not abruptly discontinue BUTRANS in a physically dependent patient because rapid discontinuation of opioid analgesics has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. (2.5)
Dosage Forms and Strengths
Transdermal system: 5 mcg/hour, 7.5 mcg/hour, 10 mcg/hour, 15 mcg/hour, and 20 mcg/hour. (3)
Contraindications
- Significant respiratory depression (4)
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment (4)
- Known or suspected gastrointestinal obstruction, including paralytic ileus (4)
- Hypersensitivity to buprenorphine (4)
Warnings and Precautions
- Life Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration. (5.6)
- Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. (5.7)
- Severe Hypotension: Monitor during dose initiation and titration. Avoid use of BUTRANS in patients with circulatory shock (5.8)
- Risks of Use in Patients with Increased Intracranial Cranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. Avoid use of BUTRANS in patients with impaired consciousness or coma. (5.9)
Adverse Reactions/Side Effects
Most common adverse reactions (≥ 5%) include: nausea, headache, application site pruritus, dizziness, constipation, somnolence, vomiting, application site erythema, dry mouth, and application site rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Purdue Pharma L.P. at 1-888-726-7535 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Benzodiazepines: May increase buprenorphine-induced respiratory depression. Monitor patients on concurrent therapy closely. (7)
- CYP3A4 Inhibitors/Inducers: Initiating CYP3A4 inhibitors or discontinuing CYP3A4 inducers may result in an increase in buprenorphine plasma concentrations. Closely monitor patients starting CYP3A4 inhibitors or stopping CYP3A4 inducers for respiratory depression. (7)
- Serotonergic Drugs: Concomitant use may result in serotonin syndrome. Discontinue BUTRANS if serotonin syndrome is suspected. (7)
- Mixed Agonist/Antagonist Analgesics: Avoid use with BUTRANS because they may reduce analgesic effect of BUTRANS or precipitate withdrawal symptoms. (7)
Use In Specific Populations
- Pregnancy: May cause fetal harm. (8.1)
- Lactation: Not recommended (8.2).
- Severe Hepatic Impairment: Consider use of an alternate analgesic that may permit more flexibility in dosing. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2022
Related/similar drugs
aspirin, acetaminophen, tramadol, duloxetine, naproxen, oxycodone, TylenolFull Prescribing Information
WARNING: ADDICTION, ABUSE and MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL EXPOSURE; NEONATAL OPIOID WITHDRAWAL SYNDROME; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
- complete a REMS-compliant education program,
- counsel patients and/or their caregivers, with every prescription, on safe use, serious risks, storage, and disposal of these products,
- emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist, and
- consider other tools to improve patient, household, and community safety.
- Reserve concomitant prescribing of BUTRANS and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
1. Indications and Usage for Butrans Patch
- Because of the risks of addiction, abuse and misuse with opioids, even at recommended doses, and because of the greater risk of overdose and death with extended-release opioid formulations [see Warnings and Precautions (5.1)], reserve BUTRANS for use in patients for whom alternative treatment options (e.g., non-opioid analgesics or immediate-release opioids) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain.
- BUTRANS is not indicated as an as-needed (prn) analgesic
2. Butrans Patch Dosage and Administration
2.1 Important Dosage and Administration Information
- Use the lowest effective dosage for the shortest duration consistent with individual patients treatment goals [see Warnings and Precautions (5)].
- Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1)].
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with BUTRANS [see Warnings and Precautions (5.3)].
- Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way and not to cut BUTRANS.
- Instruct patients to avoid exposing BUTRANS to external heat sources, hot water, or prolonged direct sunlight [see Warnings and Precautions (5.15)].
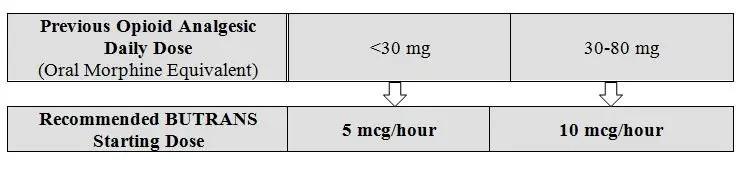
2.3 Initial Dosage
Prior Total Daily Dose of Opioid Between 30 mg to 80 mg of Oral Morphine Equivalents per Day:
2.7 Administration of BUTRANS
- Instruct patients to apply immediately after removal from the individually sealed pouch. Instruct patients not to use BUTRANS if the pouch seal is broken or the patch is cut, damaged, or changed in any way. See the Instructions for Use for step-by-step instructions for applying BUTRANS.
- Apply BUTRANS to the upper outer arm, upper chest, upper back or the side of the chest. These 4 sites (each present on both sides of the body) provide 8 possible application sites. Rotate BUTRANS among the 8 described skin sites. After BUTRANS removal, wait a minimum of 21 days before reapplying to the same skin site [see Clinical Pharmacology (12.3)].
- Apply BUTRANS to a hairless or nearly hairless skin site. If none are available, the hair at the site should be clipped, not shaven. Do not apply BUTRANS to irritated skin. If the application site must be cleaned, clean the site with water only. Do not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before applying BUTRANS.
- Incidental exposure of the BUTRANS patch to water, such as while bathing or showering is acceptable based on experience during clinical studies.
- If problems with adhesion of BUTRANS occur, the edges may be taped with first aid tape. If problems with lack of adhesion continue, the patch may be covered with waterproof or semipermeable adhesive dressings suitable for 7 days of wear.
- If BUTRANS falls off during the 7-day dosing interval, dispose of the transdermal system properly and place a new BUTRANS patch on at a different skin site.
- When changing the system, instruct patients to remove BUTRANS and dispose of it properly [see Dosage and Administration (2.8)].
- If the buprenorphine-containing adhesive matrix accidentally contacts the skin, instruct patients or caregivers to wash the area with water and not to use soap, alcohol, or other solvents to remove the adhesive because they may enhance the absorption of the drug.
3. Dosage Forms and Strengths
- BUTRANS 5 mcg/hour Transdermal System (dimensions: 45 mm by 45 mm)
- BUTRANS 7.5 mcg/hour Transdermal System (dimensions: 58 mm by 45 mm)
- BUTRANS 10 mcg/hour Transdermal System (dimensions: 45 mm by 68 mm)
- BUTRANS 15 mcg/hour Transdermal System (dimensions: 59 mm by 72 mm)
- BUTRANS 20 mcg/hour Transdermal System (dimensions: 72 mm by 72 mm)
4. Contraindications
BUTRANS is contraindicated in patients with:
- Significant respiratory depression [see Warnings and Precautions (5.3)]
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see Warnings and Precautions (5.6)]
- Known or suspected gastrointestinal obstruction, including paralytic ileus [see Warnings and Precautions (5.16)]
- Hypersensitivity (e.g., anaphylaxis) to buprenorphine [see Warnings and Precautions (5.13) and Adverse Reactions (6)]
5. Warnings and Precautions
5.2 Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
- Complete a REMS-compliant education program offered by an accredited provider of continuing education (CE) or another education program that includes all the elements of the FDA Education Blueprint for Health Care Providers Involved in the Management or Support of Patients with Pain.
- Discuss the safe use, serious risks, and proper storage and disposal of opioid analgesics with patients and/or their caregivers every time these medicines are prescribed. The Patient Counseling Guide (PCG) can be obtained at this link: www.fda.gov/OpioidAnalgesicREMSPCG.
- Emphasize to patients and their caregivers the importance of reading the Medication Guide that they will receive from their pharmacist every time an opioid analgesic is dispensed to them.
- Consider using other tools to improve patient, household, and community safety, such as patient-prescriber agreements that reinforce patient-prescriber responsibilities.
5.3 Life-Threatening Respiratory Depression
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
5.6 Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
5.9 Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury or Impaired Consciousness
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in the labeling:
- Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)]
- Life-Threatening Respiratory Depression [see Warnings and Precautions (5.3)]
- Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions (5.4)]
- Interactions with Benzodiazepines or Other CNS Depressants [see Warnings and Precautions (5.5)]
- Adrenal Insufficiency [see Warnings and Precautions (5.7)]
- Severe Hypotension [see Warnings and Precautions (5.9)]
- Hepatotoxicity [see Warnings and Precautions (5.10)]
- Application Site Skin Reactions [see Warnings and Precautions (5.11)]
- QTc Prolongation [see Warnings and Precautions (5.12)]
- Anaphylactic/Allergic Reactions [see Warnings and Precautions (5.13)]
- Gastrointestinal Effects [see Warnings and Precautions (5.17)]
- Seizures [see Warnings and Precautions (5.18)]
6.1 Clinical Trial Experience
| Open-Label Titration Period | Double-Blind Treatment Period | ||
| BUTRANS | BUTRANS | Placebo | |
| MedDRA Preferred Term | (N = 1024) | (N = 256) | (N = 283) |
| Nausea | 23% | 13% | 10% |
| Dizziness | 10% | 4% | 1% |
| Headache | 9% | 5% | 5% |
| Application site pruritus | 8% | 4% | 7% |
| Somnolence | 8% | 2% | 2% |
| Vomiting | 7% | 4% | 1% |
| Constipation | 6% | 4% | 1% |
| Open-Label Titration Period | Double-Blind Treatment Period | ||
| BUTRANS | BUTRANS 20 | BUTRANS 5 | |
| MedDRA Preferred Term | (N = 1160) | (N = 219) | (N = 221) |
| Nausea | 14% | 11% | 6% |
| Application site pruritus | 9% | 13% | 5% |
| Headache | 9% | 8% | 3% |
| Somnolence | 6% | 4% | 2% |
| Dizziness | 5% | 4% | 2% |
| Constipation | 4% | 6% | 3% |
| Application site erythema | 3% | 10% | 5% |
| Application site rash | 3% | 8% | 6% |
| Application site irritation | 2% | 6% | 2% |
| MedDRA Preferred Term | BUTRANS (N = 392) | Placebo (N = 261) |
| Nausea | 21% | 6% |
| Application site pruritus | 15% | 12% |
| Dizziness | 15% | 7% |
| Headache | 14% | 9% |
| Somnolence | 13% | 4% |
| Constipation | 13% | 5% |
| Vomiting | 9% | 1% |
| Application site erythema | 7% | 2% |
| Application site rash | 6% | 6% |
| Dry mouth | 6% | 2% |
| Fatigue | 5% | 1% |
| Hyperhidrosis | 4% | 1% |
| Peripheral edema | 3% | 1% |
| Pruritus | 3% | 0% |
| Stomach discomfort | 2% | 0% |
Gastrointestinal disorders: diarrhea, dyspepsia, and upper abdominal pain
Injury, poisoning and procedural complications: fall
Metabolism and nutrition disorders: anorexia
Nervous system disorders: hypoesthesia, tremor, migraine, and paresthesia
Psychiatric disorders: insomnia, anxiety, and depression
Respiratory, thoracic and mediastinal disorders: dyspnea, pharyngolaryngeal pain, and cough
Skin and subcutaneous tissue disorders: pruritus, hyperhidrosis, rash, and generalized pruritus
7. Drug Interactions
Table 5 Includes clinically significant drug interactions with BUTRANS.
| Benzodiazepines | |
| Clinical Impact: | There have been a number of reports regarding coma and death associated with the misuse and abuse of the combination of buprenorphine and benzodiazepines. In many, but not all of these cases, buprenorphine was misused by self-injection of crushed buprenorphine tablets. Preclinical studies have shown that the combination of benzodiazepines and buprenorphine altered the usual ceiling effect on buprenorphine-induced respiratory depression, making the respiratory effects of buprenorphine appear similar to those of full opioid agonists. |
| Intervention: | Closely monitor patients with concurrent use of BUTRANS and benzodiazepines. Warn patients that it is extremely dangerous to self-administer benzodiazepines while taking BUTRANS, and warn patients to use benzodiazepines concurrently with BUTRANS only as directed by their physician. |
| Benzodiazepines and Other Central Nervous System (CNS) Depressants | |
| Clinical Impact: | Due to additive pharmacologic effects, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, can increase the risk of hypotension, respiratory depression, profound sedation, coma, and death. |
| Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation. If concomitant use is warranted, consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration (2.2), Warnings and Precautions (5.1, 5.3, 5.5), Overdosage (10)]. |
| Examples: | Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol. |
| Inhibitors of CYP3A4 | |
| Clinical Impact: | The concomitant use of buprenorphine
and CYP3A4 inhibitors can increase the plasma concentration of buprenorphine,
resulting in increased or prolonged opioid effects, particularly when
an inhibitor is added after a stable dose of BUTRANS is achieved. After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the buprenorphine plasma concentration will decrease [see Clinical Pharmacology (12.3)], potentially resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to buprenorphine. |
| Intervention: | If concomitant use is necessary,
consider dosage reduction of BUTRANS until stable drug effects are
achieved. Monitor patients for respiratory depression and sedation
at frequent intervals. If a CYP3A4 inhibitor is discontinued, consider increasing the BUTRANS dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. |
| Examples: | Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g. ketoconazole), protease inhibitors (e.g., ritonavir) |
| CYP3A4 Inducers | |
| Clinical Impact: | The concomitant use of buprenorphine
and CYP3A4 inducers can decrease the plasma concentration of buprenorphine [see Clinical Pharmacology (12.3)], potentially resulting in decreased efficacy or onset
of a withdrawal syndrome in patients who have developed physical dependence
to buprenorphine. After stopping a CYP3A4 inducer, as the effects of the inducer decline, the buprenorphine plasma concentration will increase [see Clinical Pharmacology (12.3)], which could increase or prolong both therapeutic effects and adverse reactions and may cause serious respiratory depression. |
| Intervention: | If concomitant use is necessary,
consider increasing the BUTRANS dosage until stable drug effects are
achieved. Monitor for signs of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider BUTRANS dosage reduction and monitor for signs of respiratory depression. |
| Examples: | Rifampin, carbamazepine, phenytoin |
| Serotonergic Drugs | |
| Clinical Impact: | The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. |
| Intervention: | If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue BUTRANS if serotonin syndrome is suspected. |
| Examples: | Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). |
| Monoamine Oxidase Inhibitors (MAOIs) | |
| Clinical Impact: | MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.3)] |
| Intervention: | The use of BUTRANS is not recommended for patients taking MAOIs or within 14 days of stopping such treatment. |
| Examples: | phenelzine, tranylcypromine, linezolid |
| Mixed Agonist/Antagonist Opioid Analgesics | |
| Clinical Impact: | May reduce the analgesic effect of BUTRANS and/or precipitate withdrawal symptoms. |
| Intervention: | Avoid concomitant use. |
| Examples: | butorphanol, nalbuphine, pentazocine |
| Muscle Relaxants | |
| Clinical Impact: | Buprenorphine may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. |
| Intervention: | Monitor patients receiving muscle relaxants and BUTRANS for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of BUTRANS and/or the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration (2.2), Warnings and Precautions (5.3, 5.5), Overdosage (10)] |
| Diuretics | |
| Clinical Impact: | Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. |
| Intervention: | Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. |
| Anticholinergic Drugs | |
| Clinical Impact: | The concomitant use of opioid analgesics, including buprenorphine, and anticholinergic drugs may increase the risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. |
| Intervention: | Monitor patients for signs of urinary retention or reduced gastric motility when BUTRANS is used concomitantly with anticholinergic drugs. |
8. Use In Specific Populations
11. Butrans Patch Description
| Buprenorphine Delivery Rate (mcg/hour) | Active Surface Area (cm2) | Total Buprenorphine Content (mg) |
| BUTRANS 5 | 6.25 | 5 |
| BUTRANS 7.5 | 9.375 | 7.5 |
| BUTRANS 10 | 12.5 | 10 |
| BUTRANS 15 | 18.75 | 15 |
| BUTRANS 20 | 25 | 20 |
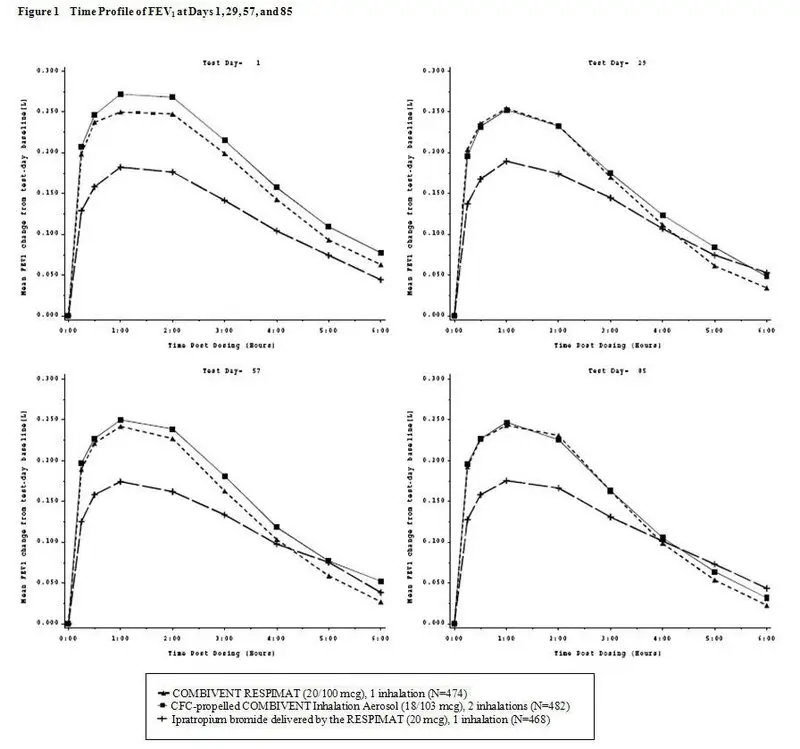
12. Butrans Patch - Clinical Pharmacology
12.3 Pharmacokinetics

Table 7: Pharmacokinetic Parameters of BUTRANS in Healthy Subjects, Mean (%CV)
| Single 7-day Application | AUCinf
(pg.h/mL) | Cmax
(pg/mL) |
| BUTRANS 5 mcg/hour | 12087 (37) | 176 (67) |
| BUTRANS 10 mcg/hour | 27035 (29) | 191 (34) |
| BUTRANS 20 mcg/hour | 54294 (36) | 471 (49) |
| Multiple 7-day Applications | AUCtau,ss
(pg.h/mL) | Cmax,ss
(pg/mL) |
| BUTRANS 10 mcg/hour, steady-state | 27543 (33) | 224 (35) |
Metabolism
Buprenorphine metabolism in the skin following BUTRANS
application is negligible.
The total clearance of buprenorphine is approximately 55 L/hour in postoperative patients.
Renal Impairment
No studies in patients with renal impairment have been
performed with BUTRANS.
16. How is Butrans Patch supplied
Store BUTRANS securely and dispose of properly [see Patient Counseling Information (17)].
Store at 25°C (77°F); excursions permitted between 15°C - 30°C (59°F - 86°F).
17. Patient Counseling Information
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Educate patients and caregivers on how to recognize the signs and symptoms of an overdose
If naloxone is prescribed, also advise patients and caregivers:
- How to treat with naloxone in the event of an opioid overdose
- To tell family and friends about their naloxone and to keep it in a place where family and friends can access it in an emergency
- To read the Patient Information (or other educational material) that will come with their naloxone. Emphasize the importance of doing this before an opioid emergency happens, so the patient and caregiver will know what to do.
Important Administration Instructions
Instruct patients how to properly use BUTRANS, including the following:
- To carefully follow instructions for the application, removal, and disposal of BUTRANS. Each week, apply BUTRANS to a different site based on the 8 described skin sites, with a minimum of 3 weeks between applications to a previously used site [see Dosage and Administration (2.7)].
- To apply BUTRANS to a hairless or nearly hairless skin site. If none are available, instruct patients to clip the hair at the site and not to shave the area. Instruct patients not to apply to irritated skin. If the application site must be cleaned, use clear water only. Soaps, alcohol, oils, lotions, or abrasive devices should not be used. Allow the skin to dry before applying BUTRANS [see Dosage and Administration (2.7)].
- To avoid exposing the BUTRANS application site to external heat sources, hot water, or prolonged direct sunlight [see Warnings and Precautions (5.15)].
Medication Guide
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 03/2021 |
| Medication
Guide BUTRANS® (BYOO-trans) (buprenorphine) Transdermal System, CIII |
|
BUTRANS is:
|
|
Important
information about BUTRANS:
|
|
Do not use
BUTRANS if you have:
|
|
Before applying
BUTRANS, tell your healthcare provider if you have a history of:
|
|
When using
BUTRANS:
|
|
While using
BUTRANS DO NOT:
|
|
The possible side
effects of BUTRANS are:
|
|
Get emergency
medical help or call 911 right away if you have:
Distributed by: Purdue Pharma L.P., Stamford, CT 06901-3431, www.purduepharma.com or call 1-888-726-7535 |
|
Instructions for Use
BUTRANS® (BYOO-trans) CIII
(buprenorphine)
Transdermal System
- Do not use soap, alcohol, lotions, oils, or other products to remove any leftover adhesive from a patch because this may cause more BUTRANS to pass through the skin.
- Each patch is sealed in its own protective pouch. Do not remove a patch from the pouch until you are ready to use it.
- Do not use a patch if the seal on the protective pouch is broken or if the patch is cut, damaged or changed in any way.
- BUTRANS patches are available in different strengths and patch sizes. Make sure you have the right strength patch that has been prescribed for you.
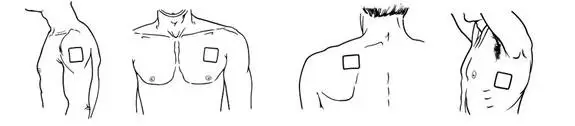
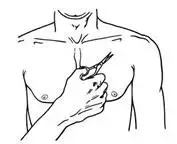
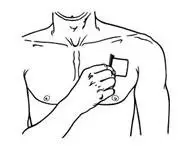
- BUTRANS should be applied to the upper outer arm, upper chest, upper back, or the side of the chest (See Figure A). These 4 sites (located on both sides of the body) provide 8 possible BUTRANS application sites.
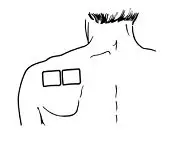
- Do not apply more than 1 patch at the same time unless your doctor tells you to. However, if your healthcare provider tells you to do so, you may use 2 patches as prescribed, applied at the same site (See Figure A for application sites) right next to each other (See Figure B for an example of patch position when applying 2 patches). Always apply and remove the two patches together at the same time.
- You should change the skin site where you apply BUTRANS each week, making sure that at least 3 weeks (21 days) pass before you re-use the same skin site.
- Apply BUTRANS to a hairless or nearly hairless skin site. If needed, you can clip the hair at the skin site (See Figure C). Do not shave the area. The skin site should not be irritated. Use only water to clean the application site. You should not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before you apply the patch.
- The skin site should be free of cuts and irritation (rashes, swelling, redness, or other skin problems).
- When you apply a new patch, write down the date and time that the patch is applied. Use this to remember when the patch should be removed.
- Change the patch at the same time of day, one week (exactly 7 days) after you apply it.
- After removing and disposing of the patch, write down the time it was removed and how it was disposed.
- If you are wearing a patch, remember to remove it before applying a new one.
- Each patch is sealed in its own protective pouch.
- If you are using two patches, remember to apply them at the same site right next to each other. Always apply and remove the two patches together at the same time.
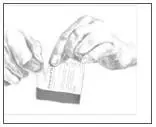
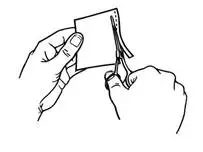
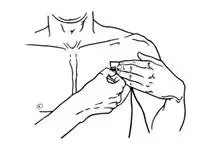
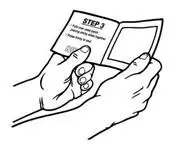
- Use scissors to cut open the pouch along the dotted line (See Figure D) and remove the patch. Do not remove the patch from the pouch until you are ready to use it. Do not use patches that have been cut or damaged in any way.
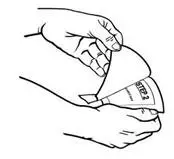
- Hold the patch with the protective liner facing you.
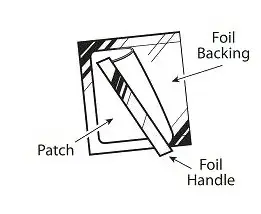
- Gently bend the patch (See Figures E and F) along the faint line and slowly peel the larger portion of the liner, which covers the sticky surface of the patch.
- Do not touch the sticky side of the patch with your fingers.
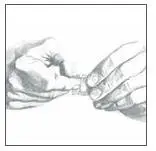
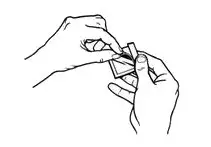
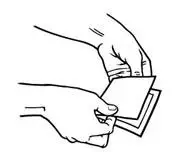
- Using the smaller portion of the protective liner as a handle (See Figure G), apply the sticky side of the patch to one of the 8 body locations described above (See “Where to apply BUTRANS”).
- While still holding the sticky side down, gently fold back the smaller portion of the patch. Grasp an edge of the remaining protective liner and slowly peel it off (See Figure H).
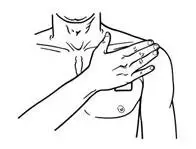
- Press the entire patch firmly into place with the palm (See Figure I) of your hand over the patch, for about 15 seconds. Do not rub the patch.
- Make sure that the patch firmly sticks to the skin.
- Go over the edges with your fingers to assure good contact around the patch.
- If you are using two patches, follow the steps in this section to apply them right next to each other.
- Always wash your hands after applying or handling a patch.
- After the patch is applied, write down the date and time that the patch is applied. Use this to remember when the patch should be removed.
Short-term exposure of the BUTRANS patch to water, such as when bathing or showering, is permitted.
If the edges of the BUTRANS patch start to loosen:
- Apply first aid tape only to the edges of the patch.
- If problems with the patch not sticking continue, cover
the patch with special see-through adhesive dressings (for example
Bioclusive or Tegaderm).
- Remove the backing from the transparent adhesive dressing and place it carefully and completely over the BUTRANS patch, smoothing it over the patch and your skin.
- Never cover a BUTRANS patch with any other bandage or tape. It should only be covered with a special see-through adhesive dressing. Talk to your healthcare provider or pharmacist about the kinds of dressing that should be used.
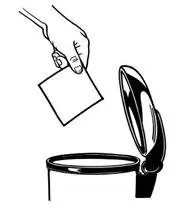
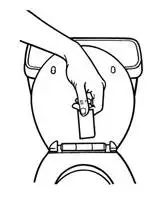
1. Peel back the disposal unit liner to show the sticky surface (See Figure K).
Never put used BUTRANS patches in the trash without first sealing them in the Patch-Disposal Unit.
This “Instructions for Use” has been approved by the U.S. Food and Drug Administration.
Distributed by: Purdue Pharma L.P., Stamford, CT 06901-3431
Revised: October
2019
©2019, Purdue Pharma L.P.
Bioclusive is a trademark of Systagenix Wound Management
(US), Inc.
Tegaderm is a trademark of 3M.
| BUTRANS
buprenorphine patch, extended release |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BUTRANS
buprenorphine patch, extended release |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BUTRANS
buprenorphine patch, extended release |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BUTRANS
buprenorphine patch, extended release |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| BUTRANS
buprenorphine patch, extended release |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Purdue Pharma LP (932323652) |
| Registrant - Purdue Pharma LP (932323652) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lohman Therapie System | 787660513 | MANUFACTURE(59011-757, 59011-750, 59011-752, 59011-751, 59011-758) | |