Drug Detail:Byfavo (Remimazolam [ rem-i-maz-oh-lam ])
Drug Class: Benzodiazepines
Highlights of Prescribing Information
BYFAVO® (remimazolam) for injection, for intravenous use, CIV
Initial U.S. Approval: 2020
WARNING: PERSONNEL AND EQUIPMENT FOR MONITORING AND RESUSCITATION, AND RISKS FROM CONCOMITANT USE WITH OPIOID ANALGESICS AND OTHER SEDATIVE-HYPNOTICS
See full prescribing information for complete boxed warning
- Only personnel trained in the administration of procedural sedation, and not involved in the conduct of the diagnostic or therapeutic procedure, should administer BYFAVO. (2.1, 5.1)
- Administering personnel must be trained in the detection and management of airway obstruction, hypoventilation, and apnea, including the maintenance of a patent airway, supportive ventilation, and cardiovascular resuscitation. (2.1, 5.1)
- BYFAVO has been associated with hypoxia, bradycardia, and hypotension. Continuously monitor vital signs during sedation and through the recovery period. (2.1, 5.1)
- Resuscitative drugs, and age- and size-appropriate equipment for bag/valve/mask assisted ventilation must be immediately available during administration of BYFAVO. (2.1, 5.1)
- Concomitant use of benzodiazepines with opioid analgesics may result in profound sedation, respiratory depression, coma, and death. The sedative effect of intravenous BYFAVO can be accentuated by concomitantly administered CNS depressant medications, including other benzodiazepines and propofol. Continuously monitor patients for respiratory depression and depth of sedation. (5.2, 7.1)
Recent Major Changes
| Warnings and Precautions (5.4) | 01/2023 |
Indications and Usage for Byfavo
BYFAVO (remimazolam) for injection is a benzodiazepine indicated for the induction and maintenance of procedural sedation in adults undergoing procedures lasting 30 minutes or less. (1)
Byfavo Dosage and Administration
Individualize and titrate BYFAVO dosing to desired clinical effect. (2.2)
Adult Patients:
- Administer an initial dose intravenously as a 5 mg push injection over a 1-minute time period. (2.2)
- If necessary, administer supplemental doses of 2.5 mg intravenously over a 15-second time period. At least 2 minutes must elapse prior to the administration of any supplemental dose. (2.2)
ASA III-IV Patients (at the discretion of the physician):
- Based on the general condition of the patient, administer 2.5 mg to 5 mg over 1-minute time period. (2.2)
- If necessary, administer supplemental doses of 1.25 mg to 2.5 mg intravenously over a 15-second time period. At least 2 minutes must elapse prior to the administration of any supplemental dose. (2.2)
Dosage Forms and Strengths
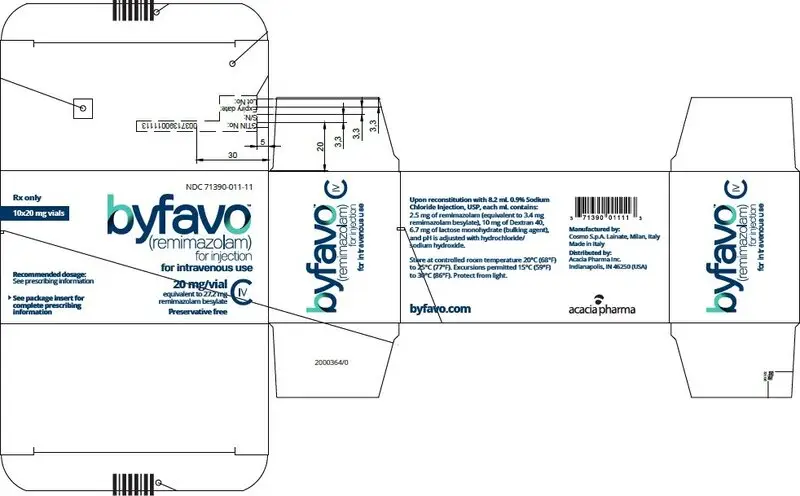
Each glass, single-patient-use vial contains 20 mg BYFAVO (remimazolam) lyophilized powder for reconstitution, equivalent to 27.2 mg remimazolam besylate. (3)
Contraindications
Hypersensitivity to dextran 40. (4)
Warnings and Precautions
Hypersensitivity Reactions: Hypersensitivity reactions including anaphylaxis may occur. (5.3)
Neonatal Sedation and Withdrawal Syndrome: Receiving benzodiazepines during pregnancy can result in neonatal sedation and/or neonatal withdrawal. (5.4, 8.1)
Pediatric Neurotoxicity: In developing animals, exposures greater than 3 hours cause neurotoxicity. Weigh benefits against potential risks when considering elective procedures in children under 3 years old. (5.5)
Adverse Reactions/Side Effects
The most common adverse reactions (>10%) in patients receiving BYFAVO for procedural sedation are hypotension, hypertension, diastolic hypertension, systolic hypertension, hypoxia, and diastolic hypotension. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Acacia Pharma at 1-877-357-9237 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Use In Specific Populations
Lactation: A lactating woman may pump and discard breast milk for 5 hours after treatment with BYFAVO. (8.2)
Pediatric Use: BYFAVO should not be used in patients less than 18 years of age. (8.4)
Geriatric Use: Sedating drugs, such as BYFAVO, may cause confusion and over-sedation in the elderly; elderly patients generally should be observed closely. (8.5)
Severe Hepatic Impairment: In patients with severe hepatic impairment the dose of BYFAVO should be carefully titrated to effect. Depending on the overall status of the patient, reduced doses might be indicated. (8.6, 12.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2023
Full Prescribing Information
WARNING: PERSONNEL AND EQUIPMENT FOR MONITORING AND RESUSCITATION AND RISKS FROM CONCOMITANT USE WITH OPIOID ANALGESICS
1. Indications and Usage for Byfavo
BYFAVO® is indicated for the induction and maintenance of procedural sedation in adults undergoing procedures lasting 30 minutes or less.
2. Byfavo Dosage and Administration
2.1 Important Dosage and Administration Instructions
BYFAVO can depress respiration. Continuously monitor patients for early signs of hypoventilation, airway obstruction, and apnea using capnography, pulse oximetry, and clinical assessment.
Only personnel trained in the administration of procedural sedation, and not involved in the conduct of the diagnostic or therapeutic procedure, should administer BYFAVO.
Administering personnel must be trained in the detection and management of airway obstruction, hypoventilation, and apnea, including the maintenance of a patent airway, supportive ventilation, and cardiovascular resuscitation.
Supplemental oxygen, resuscitative drugs, and age- and size-appropriate equipment for bag/valve/mask assisted ventilation must be immediately available during administration of BYFAVO. A benzodiazepine reversal agent should be immediately available.
Continuously monitor vital signs during sedation and through the recovery period [see Warnings and Precautions (5.1)].
Peak sedation occurs approximately 3 to 3.5 minutes after an initial 5 mg intravenous injection of BYFAVO given over a 1-minute period [see Clinical Pharmacology (12.2)].
Titrate subsequent doses of BYFAVO on the basis of clinical judgment and assessment of the depth of sedation. If maintenance of procedural sedation is inadequate, consider alternative medications [see Clinical Studies (14)].
2.2 Basic Dosing Information
- Individualize BYFAVO dosing and titrate to desired clinical response.
- In clinical studies, fentanyl 25 to 75 mcg was administered for analgesia prior to the first dose of BYFAVO. Supplemental doses of fentanyl were administered as needed for analgesia [see Clinical Studies (14)].
- Recommended dosing guidelines:
|
|
| Induction of Procedural Sedation | For adult patients: Administer 5 mg intravenously over a 1-minute time period. |
| For ASA* III and IV patients: Administer 2.5 mg to 5 mg intravenously over 1 minute based on the general condition of the patient. | |
| Maintenance of Procedural Sedation (as needed) | For adult patients: Administer 2.5 mg intravenously over 15 seconds. At least 2 minutes must elapse prior to administration of any supplemental dose. |
| For ASA III and IV patients: Administer 1.25 mg to 2.5 mg intravenously over 15 seconds. At least 2 minutes must elapse prior to administration of any supplemental dose. |
|
2.4 Administration with Other Fluids
-
BYFAVO has been shown to be compatible with the following intravenous fluids:
- 0.9% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP
- 20% Dextrose Injection, USP
- 5% Dextrose and 0.45% Sodium Chloride Injection, USP.
- BYFAVO has also been shown to be compatible with Ringer's Solution, a solution containing Sodium Chloride, Potassium Chloride and Calcium Chloride Dihydrate.
- BYFAVO has been shown to be incompatible with the following intravenous fluids:
- Lactated Ringer's Solution, a solution containing Sodium Chloride, Sodium Lactate, Potassium Chloride, and Calcium Chloride Dihydrate. Lactated Ringer's Solution is also known as Ringer's Lactate Solution, Compound Sodium Lactate Solution, and Hartmann's Solution.
- Acetated Ringer's Solution, a solution containing Sodium Chloride, Sodium Acetate, Potassium Chloride, and Calcium Chloride Dihydrate.
- BYFAVO compatibility with other agents has not been adequately evaluated.
- Do not mix BYFAVO with other drugs or fluids prior to administration.
3. Dosage Forms and Strengths
Single-patient-use vial: Each glass, single-patient-use vial of BYFAVO (remimazolam) for injection contains 20 mg remimazolam white to off-white lyophilized powder, equivalent to 27.2 mg remimazolam besylate.
4. Contraindications
BYFAVO is contraindicated in patients with a history of severe hypersensitivity reaction to dextran 40 or products containing dextran 40 [see Warnings and Precautions (5.3)].
5. Warnings and Precautions
5.1 Personnel and Equipment for Monitoring and Resuscitation
Clinically notable hypoxia, bradycardia, and hypotension were observed in Phase 3 studies of BYFAVO. Continuously monitor vital signs during sedation and through the recovery period.
Only personnel trained in the administration of procedural sedation, and not involved in the conduct of the diagnostic or therapeutic procedure, should administer BYFAVO.
Administering personnel must be trained in the detection and management of airway obstruction, hypoventilation, and apnea, including the maintenance of a patent airway, supportive ventilation, and cardiovascular resuscitation.
Resuscitative drugs, and age- and size-appropriate equipment for bag/valve/mask assisted ventilation must be immediately available during administration of BYFAVO [see Dosage and Administration (2.1)].
Consider the potential for worsened cardiorespiratory depression prior to using BYFAVO concomitantly with other drugs that have the same potential (e.g., opioid analgesics or other sedative-hypnotics) [see Drug Interactions (7.1)].
Administer supplemental oxygen to sedated patients through the recovery period.
A benzodiazepine reversal agent (flumazenil) should be immediately available during administration of BYFAVO [see Overdosage (10)].
5.2 Risks from Concomitant Use with Opioid Analgesics and Other Sedative-Hypnotics
Concomitant use of benzodiazepines, including BYFAVO, and opioid analgesics may result in profound sedation, respiratory depression, coma, and death [see Drug Interactions (7.1)].
The sedative effect of intravenous BYFAVO can be accentuated by concomitantly administered CNS depressant medications, including other benzodiazepines and propofol.
Titrate the dose of BYFAVO when administered with opioid analgesics and sedative-hypnotics to the desired clinical response.
Continuously monitor sedated patients for hypotension, airway obstruction, hypoventilation, apnea, and oxygen desaturation. These cardiorespiratory effects may be more likely to occur in patients with obstructive sleep apnea, the elderly, and ASA III or IV patients.
5.3 Hypersensitivity Reactions
BYFAVO contains dextran 40, which can cause hypersensitivity reactions, including rash, urticaria, pruritus, and anaphylaxis. BYFAVO is contraindicated in patients with a history of severe hypersensitivity reaction to dextran 40 or products containing dextran 40 [see Contraindications (4), Adverse Reactions (6)].
5.4 Neonatal Sedation and Withdrawal Syndrome
Receiving benzodiazepines late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate. Monitor neonates exposed to benzodiazepines, including BYFAVO, during pregnancy or labor for signs of sedation and monitor neonates exposed to benzodiazepines during pregnancy for signs of withdrawal and manage these neonates accordingly [see Use in Specific Populations (8.1)].
5.5 Pediatric Neurotoxicity
Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours.
The clinical significance of these findings is not clear. However, based on the available data, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately three years of age in humans [see Use in Specific Populations (8.1, 8.4), Nonclinical Pharmacology (13.2)].
Some published studies in children suggest that similar deficits may occur after repeated or prolonged exposures to anesthetic agents early in life and may result in adverse cognitive or behavioral effects. These studies have substantial limitations, and it is not clear if the observed effects are due to the anesthetic/sedation drug administration or other factors such as the surgery or underlying illness.
Anesthetic and sedation drugs are a necessary part of the care of children needing surgery, other procedures, or tests that cannot be delayed, and no specific medications have been shown to be safer than any other. Decisions regarding the timing of any elective procedures requiring anesthesia should take into consideration the benefits of the procedure weighed against the potential risks.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections:
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.4), Use in Specific Populations (8.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of BYFAVO was evaluated in three prospective, randomized, double-blind, multicenter, parallel group clinical studies in 630 patients undergoing colonoscopy (two studies) or bronchoscopy (one study). Colonoscopy Study 1 and the bronchoscopy study evaluated American Society of Anesthesiologists (ASA) physical status I to III patients, and Colonoscopy Study 2 evaluated ASA III and IV patients.
All three studies evaluated the safety of BYFAVO compared to placebo with midazolam rescue and an open-label midazolam treatment arm. Patients were administered a total dose ranging from 5 to 30 mg of BYFAVO. In these studies, the most common adverse reactions (incidence greater than 10%) following BYFAVO administration were hypotension, hypertension, diastolic hypertension, systolic hypertension, hypoxia, and diastolic hypotension. There were two patients who experienced an adverse reaction that led to discontinuation of study drug. One patient in the BYFAVO arm in the bronchoscopy study discontinued treatment due to bradycardia, hypertension, hypotension, hypoxia, and respiratory rate increase. One patient in the open-label midazolam arm in Colonoscopy Study 2 discontinued due to respiratory acidosis. No deaths were reported during the studies.
Tables 1-3 provide a summary of the common adverse reactions observed in each of the three Phase 3 studies with BYFAVO.
| Adverse Reaction | BYFAVO N = 296 | Placebo (with Midazolam Rescue*) N = 60 | Midazolam N = 102 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
|
|||
| Hypotension† | 115 (39%) | 25 (42%) | 63 (62%) |
| Hypertension‡ | 59 (20%) | 17 (28%) | 18 (18%) |
| Bradycardia | 33 (11%) | 7 (12%) | 16 (16%) |
| Diastolic hypertension‡ | 29 (10%) | 6 (10%) | 9 (9%) |
| Tachycardia | 23 (8%) | 7 (12%) | 13 (13%) |
| Diastolic hypotension† | 23 (8%) | 4 (7%) | 9 (9%) |
| Systolic hypertension‡ | 16 (5%) | 5 (8%) | 6 (6%) |
| Adverse Reaction | BYFAVO N = 303 | Placebo (with Midazolam Rescue*) N = 59 | Midazolam N = 69 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
|
|||
| Hypotension† | 99 (33%) | 28 (47%) | 23 (33%) |
| Hypertension‡ | 85 (28%) | 9 (15%) | 19 (28%) |
| Diastolic hypertension‡ | 77 (25%) | 15 (25%) | 16 (23%) |
| Systolic hypertension‡ | 67 (22%) | 13 (22%) | 17 (25%) |
| Hypoxia | 66 (22%) | 12 (20%) | 13 (19%) |
| Respiratory rate increased | 43 (14%) | 6 (10%) | 10 (14%) |
| Diastolic hypotension† | 41 (14%) | 17 (29%) | 16 (23%) |
| Nausea | 12 (4%) | 2 (3%) | 2 (3%) |
| Bradycardia | 11 (4%) | 4 (7%) | 4 (6%) |
| Pyrexia | 11 (4%) | 1 (2%) | 1 (1%) |
| Headache | 8 (3%) | 0 (0%) | 3 (4%) |
| Adverse Reaction | BYFAVO N = 31 | Placebo (with Midazolam Rescue*) N = 16 | Midazolam N = 30 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
|
|||
| Hypotension† | 18 (58%) | 11 (69%) | 17 (57%) |
| Hypertension‡ | 13 (42%) | 6 (38%) | 13 (43%) |
| Respiratory acidosis | 6 (19%) | 2 (13%) | 8 (27%) |
| Diastolic hypertension‡ | 3 (10%) | 0 (0%) | 0 (0%) |
| Systolic hypertension‡ | 2 (6%) | 0 (0%) | 0 (0%) |
| Bradycardia | 1 (3%) | 1 (6%) | 4 (13%) |
| Respiratory rate decreased | 1 (3%) | 1 (6%) | 2 (7%) |
| Diastolic hypotension† | 1 (3%) | 1 (6%) | 0 (0%) |
| Blood pressure diastolic increased | 1 (3%) | 0 (0%) | 0 (0%) |
| Blood pressure increased | 1 (3%) | 0 (0%) | 0 (0%) |
| Blood pressure systolic increased | 1 (3%) | 0 (0%) | 0 (0%) |
| Upper respiratory tract infection | 1 (3%) | 0 (0%) | 0 (0%) |
Adverse reaction data from Colonoscopy Study 1 and the bronchoscopy study analyzed according to the cumulative dose of concomitant fentanyl (<100 mcg, 100-150 mcg and >150 mcg) suggest an increase in some adverse reactions with increasing fentanyl dose, such as hypotension, hypertension, bradycardia, hypoxia, and increased respiratory rate (see Table 4 and Table 5). There were too few patients in each fentanyl stratum in Colonoscopy Study 2 to perform this analysis.
| BYFAVO | Placebo (with Midazolam Rescue†) | Midazolam | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fentanyl dose (mcg) | <100 | 100-150 | >150 | <100 | 100-150 | >150 | <100 | 100-150 | >150 |
| N = 148 | N = 146 | N = 2 | N = 9 | N = 43 | N = 8 | N = 31 | N = 62 | N = 9 | |
| Adverse Reaction | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
|
|||||||||
| Hypotension‡ | 49 (33%) | 64 (44%) | 2 (100%) | 5 (56%) | 17 (40%) | 3 (38%) | 18 (58%) | 36 (58%) | 9 (100%) |
| Hypertension§ | 24 (16%) | 35 (24%) | 0 (0%) | 1 (11%) | 14 (33%) | 2 (25%) | 3 (10%) | 12 (19%) | 3 (33%) |
| Bradycardia | 12 (8%) | 20 (14%) | 1 (50%) | 0 (0%) | 5 (12%) | 2 (25%) | 1 (3%) | 13 (21%) | 2 (22%) |
| Diastolic hypertension§ | 9 (6%) | 20 (14%) | 0 (0%) | 0 (0%) | 3 (7%) | 3 (38%) | 2 (6%) | 7 (11%) | 0 (0%) |
| Tachycardia | 10 (7%) | 12 (8%) | 1 (50%) | 0 (0%) | 6 (14%) | 1 (13%) | 2 (6%) | 8 (13%) | 3 (33%) |
| Diastolic hypotension‡ | 10 (7%) | 13 (9%) | 0 (0%) | 0 (0%) | 3 (7%) | 1 (13%) | 3 (10%) | 4 (6%) | 2 (22%) |
| Systolic hypertension§ | 5 (3%) | 11 (8%) | 0 (0%) | 0 (0%) | 3 (7%) | 2 (25%) | 4 (13%) | 2 (3%) | 0 (0%) |
| BYFAVO | Placebo (with Midazolam Rescue†) | Midazolam | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fentanyl dose (mcg) | <100 | 100-150 | >150 | <100 | 100-150 | >150 | <100 | 100-150 | >150 |
| N = 215 | N = 63 | N = 25 | N = 26 | N = 18 | N = 15 | N = 29 | N = 27 | N = 13 | |
| Adverse Reaction | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
|
|||||||||
| Hypotension‡ | 52 (24%) | 32 (51%) | 16 (64%) | 7 (27%) | 9 (50%) | 12 (80%) | 7 (24%) | 7 (26%) | 9 (69%) |
| Hypertension§ | 43 (20%) | 25 (40%) | 18 (72%) | 2 (8%) | 2 (11%) | 5 (33%) | 3 (10%) | 8 (30%) | 8 (62%) |
| Diastolic hypertension§ | 65 (30%) | 12 (19%) | 0 (0%) | 11 (42%) | 3 (17%) | 1 (7%) | 10 (34%) | 6 (22%) | 0 (0%) |
| Systolic hypertension§ | 55 (26%) | 11 (17%) | 1 (4%) | 10 (38%) | 3 (17%) | 0 (0%) | 9 (31%) | 6 (22%) | 2 (15%) |
| Hypoxia | 35 (16%) | 22 (35%) | 9 (36%) | 6 (23%) | 2 (11%) | 4 (27%) | 2 (7%) | 5 (19%) | 6 (46%) |
| Respiratory rate increased | 22 (10%) | 12 (19%) | 9 (36%) | 1 (4%) | 2 (11%) | 3 (20%) | 2 (7%) | 5 (19%) | 3 (23%) |
| Diastolic hypotension‡ | 28 (13%) | 13 (21%) | 0 (0%) | 8 (31%) | 7 (39%) | 2 (13%) | 7 (24%) | 6 (22%) | 3 (23%) |
| Nausea | 9 (4%) | 1 (2%) | 2 (8%) | 0 (0%) | 0 (0%) | 2 (13%) | 1 (3%) | 1 (4%) | 0 (0%) |
| Bradycardia | 3 (1%) | 4 (6%) | 4 (16%) | 2 (8%) | 1 (6%) | 1 (7%) | 0 (0%) | 2 (7%) | 2 (15%) |
| Pyrexia | 7 (3%) | 2 (3%) | 2 (8%) | 0 (0%) | 0 (0%) | 1 (7%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Headache | 5 (2%) | 2 (3%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (11%) | 0 (0)% |
7. Drug Interactions
7.1 Opioid Analgesics and Other Sedative-Hypnotics
The sedative effect of intravenous BYFAVO can be accentuated by concomitantly administered CNS depressant medications, including opioid analgesics, other benzodiazepines, and propofol. Continuously monitor vital signs during sedation and through the recovery period. Titrate the dose of BYFAVO when administered with opioid analgesics and sedative-hypnotics to the desired clinical response [see Warnings and Precautions (5.2)].
8. Use In Specific Populations
8.2 Lactation
Data
In rabbits administered daily intravenous infusions of remimazolam at 12.5 and 20 mg/kg/day from 14 days before mating until Lactation Day 30, remimazolam and the metabolite CNS7054 were present in milk samples obtained after the end of an infusion on Day 10 or 11 of lactation. Remimazolam was not quantifiable in plasma samples obtained from rabbit kits taken in the morning on Day 10 or 11 of lactation. However, metabolite CNS7054 was present at low levels in 2 of the 5 kits sampled.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. No studies are available in any pediatric population and extrapolation of adult effectiveness data to the pediatric population is not possible.
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as BYFAVO, that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss; however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in pregnant women, neonates, and young children who require procedures with the potential risks suggested by the nonclinical data [see Warnings and Precautions (5.4), Warnings and Precautions (5.5), Use in Specific Populations (8.1), Nonclinical Toxicology (13.2)].
8.5 Geriatric Use
Of the total number of subjects treated with BYFAVO in clinical studies for procedural sedation, there were 649 subjects <65 years of age, 221 subjects >65 years of age, 171 subjects between 65-74 years of age, and 50 subjects >75 years of age.
No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. Some data suggest a potential of greater sensitivity (a faster onset of loss of consciousness and a longer duration of sedation) of some older individuals.
Administer supplemental doses of BYFAVO slowly to achieve the level of sedation required for the procedure, and monitor all patients for cardiorespiratory complications.
8.6 Hepatic Impairment
In patients with severe hepatic impairment, the dose of BYFAVO should be carefully titrated to effect. Depending on the overall status of the patient, lower frequency of supplemental doses may be needed to achieve the level of sedation required for the procedure. All patients should be monitored for sedation-related cardiorespiratory complications [see Clinical Pharmacology (12.3)].
9. Drug Abuse and Dependence
9.2 Abuse
BYFAVO contains the benzodiazepine, remimazolam. Benzodiazepines are a class of sedative drugs with a known potential for abuse. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. In a human abuse potential study conducted in recreational sedative abusers (n = 39), remimazolam (5 and 10 mg, IV) produced responses on positive subjective measures such as "Drug Liking," "Overall Drug Liking," "Take Drug Again," and "Good Drug Effects" that were statistically similar to those produced by the sedative midazolam (2.5 and 5 mg), and statistically greater than the responses on these measures that were produced by placebo.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. In a monkey physical dependence study, chronic administration of remimazolam produced withdrawal signs such as tremors, muscle rigidity, restlessness, impaired motor activity, and a reduction in food consumption upon drug discontinuation. One monkey of six in this study exhibited systemic convulsions and dissociation from the environment. These behaviors are consistent with benzodiazepine withdrawal, which suggests that remimazolam produces physical dependence.
11. Byfavo Description
Each glass, single-patient-use, sterile vial of BYFAVO (remimazolam) for injection contains 20 mg remimazolam, equivalent to 27.2 mg remimazolam besylate.
Remimazolam is a benzodiazepine. Its chemical description is 4H-imidazol[1,2-a][1,4]benzodiazepine-4-propionic acid, 8-bromo-1-methyl-6-(2-pyridinyl)-(4S)-, methyl ester, benzenesulfonate (1:1). The structural formulas are shown below.
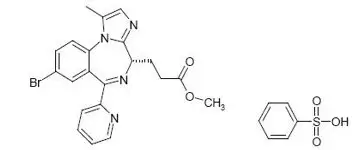
Molecular weight of BYFAVO (free base): 439.3 g/mol.
Molecular weight of BYFAVO besylate: 597.5 g/mol.
BYFAVO besylate powder is sparingly soluble in water.
BYFAVO 20 mg contains: 82 mg dextran 40 and 55 mg lactose monohydrate as bulking agents/stabilizers. The pH is adjusted with hydrochloride/sodium hydroxide. Upon reconstitution with saline, BYFAVO has a pH of 2.9 to 3.9.
12. Byfavo - Clinical Pharmacology
12.1 Mechanism of Action
BYFAVO is a benzodiazepine. BYFAVO binds to brain benzodiazepine sites (gamma amino butyric acid type A [GABAA] receptors), while its carboxylic acid metabolite (CNS7054) has a 300 times lower affinity for the receptor. BYFAVO, like other benzodiazepines, did not show clear selectivity between subtypes of the GABAA receptor.
12.2 Pharmacodynamics
Dose finding studies determined the IV dosing recommendation of the initial 5 mg bolus, followed by 2.5 mg top-up doses. Median time to peak sedation, defined as the lowest Modified Observer's Assessment of Alertness/Sedation (MOAA/S) score after the initial dose, in the Phase 3 trials was 3 to 3.5 minutes and median time to fully alert, defined as time to the first of three consecutive MOAA/S scores of five, following the last dose of BYFAVO was 11 to 14 minutes.
12.3 Pharmacokinetics
- BYFAVO has a terminal elimination half-life from plasma of 37 to 53 minutes.
- Mean distribution half-life (t1/2α) is between 0.5 and 2 minutes.
- Half-life (t1/2) is prolonged with increasing severity of hepatic impairment leading to a need for careful dose titration in patients with severe hepatic impairment.
- Clearance (54 to 75 L/h) is not related to body weight.
- In healthy subjects at least 80% and in colonoscopy patients 50% to 60% of dose is excreted in urine as inactive metabolite.
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester through the first several months of life but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of an anesthetic regimen that produced a light surgical plane of anesthesia did not increase neuronal cell loss; however, treatment regimens of 5 hours or longer increased neuronal cell loss. Data in rodents and in primates suggest that the neuronal and oligodendrocyte cell losses are associated with subtle but prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures against the potential risks suggested by the nonclinical data [See Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.4)].
14. Clinical Studies
The safety and efficacy of BYFAVO compared to a saline placebo with midazolam rescue treatment group and an open-label midazolam treatment group was evaluated in three randomized, double-blind, multicenter Phase 3 studies conducted in 969 adult patients receiving procedural sedation.
14.1 Colonoscopy Study 1 (NCT 02290873)
This Phase 3 study was conducted in 461 ASA I to III patients undergoing colonoscopy. BYFAVO 5 mg (2 mL) IV was administered as an initial bolus, followed by 2.5 mg (1 mL) top-up doses versus placebo 2 mL administered as an initial bolus, followed by 1 mL top-up doses. Midazolam rescue was dosed per investigator discretion in both treatment groups. Fentanyl was administered as an analgesic pre-treatment at an initial dose of 50 to 75 mcg IV (or a reduced dose for ASA III patients) immediately prior to administration of the initial dose of study medication. Top-up doses of fentanyl 25 mcg every 5 to 10 minutes were allowed until analgesia was adequate or a maximum dose of 200 mcg had been administered. Supplemental oxygen was administered prior to the start of the procedure and continued at a rate of 1 to 5 L/minute until the patient was fully alert after procedure completion. Colonoscopy started when adequate sedation was achieved, defined as an MOAA/S score ≤3. The primary efficacy endpoint for BYFAVO versus placebo was success of the colonoscopy procedure, defined as a composite of the following:
- Completion of the colonoscopy procedure, AND
- No requirement for a rescue sedative medication, AND
- No requirement for more than 5 doses of study medication within any 15-minute window.
There were 63 patients (13.8%) who were aged 65 years or older, 218 patients (47.6%) who were male, 339 (74.0%) who were white, 80 (17.5%) who were Black or African American, 31 (6.8%) who were Asian, and 73 (15.9%) who were Hispanic or Latino. There were 143 patients in ASA I, 285 in ASA II, and 30 in ASA III. As shown in Table 6, the colonoscopy sedation success rate was statistically significantly higher in the BYFAVO group than in the placebo group.
| Cohort | Sedation Success Rate n/N (%) |
|---|---|
| n/N = number of successes/number of subjects in group. | |
| Remimazolam | 272/298 (91.3%) |
| Placebo | 1/60 (1.7%) |
The reasons for procedural sedation failure are shown in Table 7.
| Reason | Remimazolam N = 298 n (%) | Placebo N = 60 n (%) |
|---|---|---|
| Rescue sedative medication taken | 10 (3.4%) | 57 (95%) |
| Too many doses within the predefined time window | 18 (6.0%) | 44 (73.3%) |
| Procedure not completed | 7 (2.3%) | 1 (1.7%) |
Table 8 shows the number of top-up doses required, and the total doses of study medication, fentanyl, and rescue medication administered.
| Number of Top-up Doses of Study Drug (Mean ± SD) | Total Amount of Study Drug (mg) (Mean ± SD) | Total Amount of Fentanyl (mcg) (Mean ± SD) | Total Amount of Midazolam Rescue Medication (mg) (Mean ± SD) |
|
|---|---|---|---|---|
| Remimazolam | 2.2 ± 1.6 | 10.5 ± 4.0 | 88.9 ± 21.7 | 0.3 ± 2.1 |
| Placebo | 5.1 ± 0.5 | 0 | 121.3 ± 34.4 | 6.8 ± 4.2 |
Summaries of the time to start procedure, duration of procedure, time to fully alert, and time to ready for discharge are shown in Table 9.
|
|
| Time to start procedure (minutes)* | |
| Median (95% confidence interval) | 4.0 (4.0, 4.0) |
| Min, Max | 0, 26 |
| Duration of procedure (minutes)† | |
| Median (95% confidence interval) | 12.0 (11.0, 13.0) |
| Min, Max | 3, 33 |
| Number (proportion) of procedures lasting longer than 30 minutes | 1/291 (0.3%) |
| Time to fully alert after end of colonoscopy (minutes)† | |
| Median (95% confidence interval) | 6.0 (5.0, 7.0) |
| Min, Max | 0, 44 |
| Time to ready to discharge after end of colonoscopy (minutes)† | |
| Median (95% confidence interval) | 44.0 (42.0, 46.0) |
| Min, Max | 3, 79 |
14.2 Bronchoscopy Study (NCT 02296892)
This Phase 3 study was conducted in 431 ASA I to III patients undergoing bronchoscopy. BYFAVO 5 mg (2 mL) IV was administered as an initial bolus, followed by 2.5 mg (1 mL) top-up doses versus placebo 2 mL administered as an initial bolus, followed by 1 mL top-up doses. Midazolam rescue was dosed per investigator discretion in both treatment groups. Fentanyl was administered as an analgesic pre-treatment at an initial dose of 25 to 50 mcg IV immediately prior to administration of the initial dose of study medication. Top-up doses of fentanyl 25 mcg every 5 to 10 minutes were allowed until analgesia was adequate. A maximum dose of fentanyl 200 mcg was recommended. Supplemental oxygen was administered prior to the start of the procedure and continued at a rate of 1 to 15 L/minute until the patient was fully alert after procedure completion. Bronchoscopy started when adequate sedation was achieved, defined as an MOAA/S score ≤3. The primary efficacy endpoint for BYFAVO versus placebo was successful sedation for the bronchoscopy procedure, defined as a composite of the following:
- Completion of the bronchoscopy procedure, AND
- No requirement for a rescue sedative medication, AND
- No requirement for more than 5 doses of study medication within any 15-minute window.
There were 209 patients (48.5%) who were 65 years or older, 198 patients (45.9%) who were male, 358 (83.1%) who were white, 62 (14.4%) who were Black or African American, 5 (1.2%) who were Asian, and 8 (1.9%) who were Hispanic or Latino. There were 15 patients in ASA I, 254 in ASA II, and 162 in ASA III. As shown in Table 10, the bronchoscopy sedation success rate was statistically significantly higher for the BYFAVO group than for the placebo group.
| Cohort | Total Success Rate n/N (%) |
|---|---|
| n/N = number of successes/number of subjects in group. | |
| Remimazolam | 250/310 (80.6%) |
| Placebo | 3/63 (4.8%) |
The reasons for procedural sedation failure are shown in Table 11.
| Reason | Remimazolam N = 310 n (%) | Placebo N = 63 n (%) |
|---|---|---|
| Rescue sedative medication taken | 49 (15.8%) | 57 (90.5%) |
| Too many doses within the predefined time window | 14 (4.5%) | 10 (15.9%) |
| Procedure not completed | 9 (2.9%) | 3 (4.8%) |
Table 12 shows the number of top-up doses required, and the total doses of study medication, fentanyl, and rescue medication administered.
| Number of Top-up Doses of Study Drug (Mean ± SD) | Total Amount of Study Drug (mg) (Mean ± SD) | Total Amount of Fentanyl (mcg) (Mean ± SD) | Total Amount of Midazolam Rescue Medication (mg) (Mean ± SD) |
|
|---|---|---|---|---|
| Remimazolam | 2.6 ± 2.0 | 11.5 ± 5.1 | 81.8 ± 54.3 | 1.3 ± 3.5 |
| Placebo | 4.1 ± 0.8 | 0 | 118.8 ± 79.1 | 5.9 ± 3.7 |
Summaries of the time to start procedure, duration of procedure, time to fully alert, and time to ready for discharge are shown in Table 13.
|
|
| Time to start procedure (minutes)* | |
| Median (95% confidence interval) | 4.1 (4.0, 4.8) |
| Min, Max | 1,41 |
| Duration of procedure (minutes)† | |
| Median (95% confidence interval) | 10.0 (8.0, 11.0) |
| Min, Max | 1, 68 |
| Number (proportion) of procedures lasting longer than 30 minutes† | 28/299 (9.4%) |
| Time to fully alert after end of bronchoscopy (minutes)† | |
| Median (95% confidence interval) | 6.0 (5.2, 7.1) |
| Min, Max | 1.1, 107 |
| Time to ready to discharge after end of bronchoscopy (minutes)† | |
| Median (95% confidence interval) | 60.0 (57.0, 63.0) |
| Min, Max | 6.6, 284 |
14.3 Colonoscopy Study 2 (NCT 02532647)
This Phase 3 study was conducted in 77 ASA III and IV patients undergoing colonoscopy. BYFAVO 2.5 mg (1 mL) to 5 mg (2 mL) IV was administered as an initial bolus, followed by 1.25 mg (0.5 mL) to 2.5 mg (1 mL) top-up doses versus placebo 1 to 2 mL administered with midazolam rescue, dosed per investigator discretion. Fentanyl was administered as an analgesic pre-treatment at an initial maximum dose of 50 mcg (with dose reduction for debilitated patients), immediately prior to administration of the initial dose of study medication. Top-up doses of fentanyl 25 mcg every 5 to 10 minutes were allowed until analgesia was adequate or a maximum dose of 200 mcg had been administered. Supplemental oxygen was administered prior to the start of the procedure and continued at a rate of up to 4 L/minute until the patient was fully alert after procedure completion. Colonoscopy started when adequate sedation was achieved, defined as an MOAA/S score ≤3.
The primary objective of the study was to assess the safety of multiple doses of BYFAVO compared to placebo and midazolam. Procedure success was a secondary objective and was defined as follows:
- Completion of the colonoscopy procedure, AND
- No requirement for a rescue sedative medication, AND
- No requirement for more than 5 doses of study medication within any 15-minute window.
The total patient population, including all randomized patients who received any amount of study medication, comprised 31 patients in the remimazolam group, 16 patients in the placebo group, and 30 patients in the midazolam group. There were two patients, one each in the remimazolam and midazolam treatment groups, who were randomized, but did not receive a dose of study medication.
There were 31 patients (40.2%) who were aged 65 years or older, 43 patients (55.8%) who were male, 57 (74.0%) who were white, 19 (24.7%) who were Black or African American, 1 (1.30%) who was Asian, and none who were Hispanic or Latino. There were 40 patients in ASA III and 37 patients in ASA IV.
Patients in the remimazolam group received a mean (± SD) of 9.0 (± 3.7) mg of remimazolam and a mean (± SD) of 2.5 (± 10.2) mg of midazolam compared to 7.2 (± 2.5) mg in the placebo group. The mean total dose of fentanyl was lower in the remimazolam group (mean ± SD: 59.7 ± 15.4 mcg) than in the placebo group (mean ± SD: 67.2 ± 21.8 mcg).
In the remimazolam group, 90.3% of patients did not receive any rescue sedative medication, compared to 0.0% in the placebo group.
There were no serious adverse reactions and no discontinuations due to adverse reactions observed in the remimazolam group. The incidence of hypotension (SMQ) was 61.3% in the remimazolam group and 75% in the placebo group.
No inferential statistical tests were performed in this trial. Patients who received BYFAVO for sedation during scheduled colonoscopy responded at a numerically greater rate than patients who received placebo (randomized analysis population – remimazolam: 27/32 [84.4%]; placebo: 0/16 [0%]).
16. How is Byfavo supplied
BYFAVO (remimazolam) for injection, for intravenous use is supplied as follows:
NDC 71390-011-11: Carton of 10 × 12 mL vials. Each 12 mL glass vial of BYFAVO (NDC 71390-011-00) provides a sterile lyophilized white to off-white powder intended for single-patient use only and contains 20 mg remimazolam (equivalent to 27.2 mg remimazolam besylate) ready for reconstitution.
| BYFAVO
remimazolam besylate injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Acacia Pharma, Ltd. (779660930) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cambrex Karlskoga AB | 353954043 | ANALYSIS(71390-011) , API MANUFACTURE(71390-011) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cosmo S.p.A. | 630431955 | ANALYSIS(71390-011) , MANUFACTURE(71390-011) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ITS Testing Services (UK) Ltd. (Intertek Pharmaceutical Services Manchester) | 233547491 | ANALYSIS(71390-011) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Particle Analytical ApS | 306081360 | ANALYSIS(71390-011) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Italia S.p.A. | 338336589 | ANALYSIS(71390-011) , MANUFACTURE(71390-011) , PACK(71390-011) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins BioPharma Product Testing Sweden AB | 350827847 | ANALYSIS(71390-011) | |