Drug Detail:Calcium acetate (Calcium acetate [ kal-see-um-as-e-tate ])
Drug Class: Minerals and electrolytes Phosphate binders
Highlights of Prescribing Information
CALCIUM ACETATE capsules, for oral use
Initial U.S. Approval: 1990
Indications and Usage for Calcium Acetate Capsules
- Calcium acetate capsules are a phosphate binder indicated for the reduction of serum phosphorus in patients with end stage renal disease. (1)
Calcium Acetate Capsules Dosage and Administration
- Starting dose is 2 capsules with each meal.(2)
- Titrate the dose every 2-3 weeks until acceptable serum phosphorus level is reached. Most patients require 3-4 capsules with each meal. (2)
Dosage Forms and Strengths
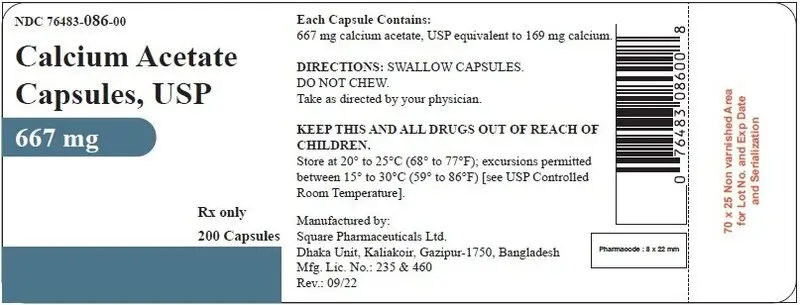
- Capsule: 667 mg calcium acetate capsule. (3)
Contraindications
- Hypercalcemia. (4)
Warnings and Precautions
- Treat mild hypercalcemia by reducing or interrupting calcium acetate and Vitamin D. Severe hypercalcemia may require hemodialysis and discontinuation of calcium acetate. (5.1)
- Hypercalcemia may aggravate digitalis toxicity. (5.2)
Adverse Reactions/Side Effects
- The most common (>10%) adverse reactions are hypercalcemia, nausea, and vomiting. (6.1)
- In clinical studies, patients have occasionally experienced nausea during calcium acetate therapy. (6)
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
- Calcium acetate may decrease the bioavailability of tetracyclines or fluoroquinolones. (7)
- When clinically significant drug interactions are expected, administer the drug at least one hour before or at least three hours after calcium acetate or consider monitoring blood levels of the drug. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
Related/similar drugs
calcium acetate, aluminum hydroxide, PhosLo, Phoslyra, AmphojelFull Prescribing Information
5. Warnings and Precautions
5.1 Hypercalcemia
An overdose of calcium acetate may lead to progressive hypercalcemia, which may require emergency measures. Therefore, early in the treatment phase during the dosage adjustment period, monitor serum calcium levels twice weekly. Should hypercalcemia develop, reduce the calcium acetate dosage, or discontinue the treatment, depending on the severity of hypercalcemia.
More severe hypercalcemia (Ca >12 mg/dL) is associated with confusion, delirium, stupor and coma. Severe hypercalcemia can be treated by acute hemodialysis and discontinuing calcium acetate therapy.
Mild hypercalcemia (10.5 to 11.9 mg/dL) may be asymptomatic or manifest as constipation, anorexia, nausea, and vomiting. Mild hypercalcemia is usually controlled by reducing the calcium acetate dose or temporarily discontinuing therapy. Decreasing or discontinuing Vitamin D therapy is recommended as well.
Chronic hypercalcemia may lead to vascular calcification and other soft-tissue calcification. Radiographic evaluation of suspected anatomical regions may be helpful in early detection of soft tissue calcification. The long term effect of calcium acetate on the progression of vascular or soft tissue calcification has not been determined.
Hypercalcemia (>11 mg/dL) was reported in 16% of patients in a 3-month study of solid dose formulation of calcium acetate; all cases resolved upon lowering the dose or discontinuing treatment.
Maintain the serum calcium-phosphorus (Ca x P) product below 55 mg2/dL2.
6. Adverse Reactions/Side Effects
Hypercalcemia is discussed elsewhere [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
In clinical studies, calcium acetate has been generally well tolerated.
Calcium acetate was studied in a 3-month, open-label, non-randomized study of 98 enrolled ESRD hemodialysis patients and an alternate liquid formulation of calcium acetate was studied in a two week double-blind, placebo-controlled, cross-over study with 69 enrolled ESRD hemodialysis patients. Adverse reactions (>2% on treatment) from these trials are presented in Table 1.
| Preferred Term
| Total adverse reactions reported for calcium acetate
n=167 n (%) | 3-mo, open-label
study of calcium acetate n=98 n (%) | Double blind, placebo-controlled, cross-over study of liquid calcium acetate
n=69 |
|
| Calcium acetate
n (%) | Placebo
n (%) |
|||
| Nausea | 6 (3.6) | 6 (6.1) | 0 (0.0) | 0 (0.0) |
| Vomiting | 4 (2.4) | 4 (4.1) | 0 (0.0) | 0 (0.0) |
| Hypercalcemia | 21 (12.6) | 16 (16.3) | 5 (7.2) | 0 (0.0) |
7. Drug Interactions
There are no empirical data on avoiding drug interactions between calcium acetate and most concomitant drugs. When administering an oral medication with calcium acetate where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, administer the drug one hour before or three hours after calcium acetate. Monitor blood levels of the concomitant drugs that have a narrow therapeutic range. Patients taking anti-arrhythmic medications for the control of arrhythmias and anti-seizure medications for the control of seizure disorders were excluded from the clinical trials with all forms of calcium acetate.
8. Use In Specific Populations
11. Calcium Acetate Capsules Description
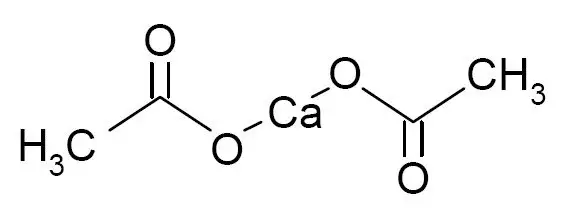
Calcium acetate capsules are administered orally for the control of hyperphosphatemia in end-stage renal failure.
"FDA approved dissolution test specifications differ from USP."
14. Clinical Studies
Ninety-one patients with end-stage renal disease who were undergoing hemodialysis and were hyperphosphatemic (serum phosphorus >5.5 mg/dL) following a 1-week phosphate binder washout period contributed efficacy data to an open-label, non-randomized study.
The patients received calcium acetate 667 mg tablets at each meal for a period of 12 weeks. The initial starting dose was 2 tablets per meal for 3 meals a day, and the dose was adjusted as necessary to control serum phosphorus levels. The average final dose after 12 weeks of treatment was 3.4 tablets per meal. Although there was a decrease in serum phosphorus, in the absence of a control group the true magnitude of effect is uncertain.
The data presented in Table 2 demonstrate the efficacy of calcium acetate in the treatment of hyperphosphatemia in end-stage renal disease patients. The effects on serum calcium levels are also presented.
| Parameter
| Pre-Study
| Week 4b
| Week 8
| Week 12
| p-valuec
|
| Phosphorus (mg/dL)a
| 7.4 ± 0.17 | 5.9 ± 0.16 | 5.6 ± 0.17 | 5.2 ± 0.17 | ≤0.01 |
| Calcium (mg/dL)a
| 8.9 ± 0.09 | 9.5 ± 0.10 | 9.7 ± 0.10 | 9.7 ± 0.10 | ≤0.01 |
| a Values expressed as mean ± SE. b Ninety-one patients completed at least 6 weeks of the study. c ANOVA of difference in values at pre-study and study completion. |
|||||
There was a 30% decrease in serum phosphorus levels during the 12 week study period (p<0.01).
Two-thirds of the decline occurred in the first month of the study. Serum calcium increased 9% during the study mostly in the first month of the study.
Treatment with the phosphate binder was discontinued for patients from the open-label study, and those patients whose serum phosphorus exceeded 5.5 mg/dL were eligible for entry into a double-blind, placebo-controlled, cross-over study. Patients were randomized to receive calcium acetate or placebo, and each continued to receive the same number of tablets as had been individually established during the previous study. Following 2 weeks of treatment, patients switched to the alternative therapy for an additional 2 weeks.
The phosphate binding effect of calcium acetate is shown in the Table 3.
| Parameter
| Pre-Study
| Post-Treatment
| p-valueb
|
|
| Calcium Acetate
| Placebo
|
|||
| Phosphorus (mg/dL)a
| 7.3 ± 0.18 | 5.9 ± 0.24 | 7.8 ± 0.22 | <0.01 |
| Calcium (mg/dL)a
| 8.9 ± 0.11 | 9.5 ± 0.13 | 8.8 ± 0.12 | <0.01 |
| a Values expressed as mean ± SEM b ANOVA of calcium acetate vs. placebo after 2 weeks of treatment. |
||||
16. How is Calcium Acetate Capsules supplied
Supplied in Bottles of 200 (NDC 76483-086-00).
STORAGE: Store at 20° to 25°C (68° to 77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
17. Patient Counseling Information
Advise patients who are taking an oral medication where reduction in the bioavailability of that medication would have clinically significant effect on its safety or efficacy to take the drug one hour before or three hours after calcium acetate.
Manufactured by:
Square Pharmaceuticals Ltd.
Dhaka Unit, Kaliakoir, Gazipur-1750,
Bangladesh
| CALCIUM ACETATE
calcium acetate capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - SQUARE PHARMACEUTICALS LIMITED (731487153) |
| Registrant - SQUARE PHARMACEUTICALS LIMITED (731487153) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SQUARE PHARMACEUTICALS LIMITED, Dhaka unit | 850366520 | ANALYSIS(76483-086) , LABEL(76483-086) , MANUFACTURE(76483-086) , PACK(76483-086) | |