Drug Detail:Cancidas (Caspofungin [ kas-poe-fun-jin ])
Drug Class: Echinocandins
Highlights of Prescribing Information
CANCIDAS® (caspofungin acetate) for injection, for intravenous use
Initial U.S. Approval: 2001
Indications and Usage for Cancidas
CANCIDAS is an echinocandin antifungal indicated in adults and pediatric patients (3 months of age and older) for:
- Empirical therapy for presumed fungal infections in febrile, neutropenic patients. (1)
- Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis and pleural space infections. (1)
- Treatment of esophageal candidiasis. (1)
- Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies. (1)
Cancidas Dosage and Administration
Important Administration Instructions for All Patients (2.1):
- Administer by slow intravenous (IV) infusion over approximately 1 hour. Do not administer by IV bolus administration.
- Do not mix or co-infuse CANCIDAS with other medications. Do not use diluents containing dextrose (α–D-glucose).
Dosage in Adults [18 years of age and older] (2.2):
- Administer a single 70-mg loading dose on Day 1, followed by 50 mg once daily for all indications except esophageal candidiasis.
- For esophageal candidiasis, use 50 mg once daily with no loading dose.
Dosage in Pediatric Patients [3 months to 17 years of age] (2.3):
- Dosing should be based on the patient's body surface area.
- For all indications, administer a single 70-mg/m2 loading dose on Day 1, followed by 50 mg/m2 once daily thereafter.
- Maximum loading dose and daily maintenance dose should not exceed 70 mg, regardless of the patient's calculated dose.
Dosage Adjustments in Patients with Hepatic Impairment (2.4):
Reduce dosage for adult patients with moderate hepatic impairment (35 mg once daily, with a 70 mg loading dose on Day 1 where appropriate).
Dosage Adjustment in Patients Receiving Concomitant Inducers of Hepatic CYP Enzymes (2.5):
- Use 70-mg once daily dose for adult patients on rifampin.
- Consider dose increase to 70 mg once daily for adult patients on nevirapine, efavirenz, carbamazepine, dexamethasone, or phenytoin.
- Pediatric patients receiving these same concomitant medications may also require an increase in dose to 70 mg/m2 once daily (maximum daily dose not to exceed 70 mg).
Dosage Forms and Strengths
- For Injection: 50 or 70 mg lyophilized powder (plus allowance for overfill) in a single-dose vial for reconstitution (3)
Contraindications
- CANCIDAS is contraindicated in patients with known hypersensitivity to any component of this product. (4)
Warnings and Precautions
- Hypersensitivity: Anaphylaxis, possible histamine-mediated adverse reactions, including rash, facial swelling, angioedema, pruritus, sensation of warmth or bronchospasm, and cases of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported with use of CANCIDAS. Discontinue CANCIDAS at the first sign or symptom of a hypersensitivity reaction and administer appropriate treatment. (5.1)
- Hepatic Effects: Can cause abnormalities in liver enzymes. Isolated cases of hepatic dysfunction, hepatitis, or hepatic failure have been reported. Monitor patients who develop abnormal liver enzymes for evidence of worsening hepatic function, and evaluate risk/benefit of continuing CANCIDAS. (5.2)
- Elevated Liver Enzymes During Concomitant Use with Cyclosporine: Limit use to patients for whom potential benefit outweighs potential risk. Monitor patients who develop abnormal liver function tests (LFTs) during concomitant use with CANCIDAS. (5.3)
Adverse Reactions/Side Effects
- Adults: Most common adverse reactions (incidence 10% or greater) are diarrhea, pyrexia, ALT/AST increased, blood alkaline phosphatase increased, and blood potassium decreased. (6.1)
- Pediatric Patients: Most common adverse reactions (incidence ≥10%) are pyrexia, diarrhea, rash, ALT/AST increased, blood potassium decreased, hypotension, and chills. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Use In Specific Populations
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Pediatric Use: Safety and efficacy in neonates and infants less than 3 months old have not been established. (8.4)
- Hepatic Impairment: Reduce dose for adult patients with moderate hepatic impairment (35 mg once daily, with a 70-mg loading dose on Day 1 where appropriate). No data are available in adults with severe impairment or in pediatric patients with any degree of hepatic impairment. (2.4, 8.6, 12.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2022
Related/similar drugs
ciprofloxacin, Augmentin, fluconazole, amoxicillin / clavulanate, vancomycin, Diflucan, gentamicinFull Prescribing Information
1. Indications and Usage for Cancidas
1.1 Empirical Therapy for Presumed Fungal Infections in Febrile, Neutropenic Patients
CANCIDAS® is indicated as empirical therapy for presumed fungal infections in febrile, neutropenic adult and pediatric patients (3 months of age and older) [see Clinical Studies (14.1, 14.5)].
1.2 Treatment of Candidemia and Other Candida Infections
CANCIDAS is indicated for the treatment of candidemia and the following candida infections: intra-abdominal abscesses, peritonitis, and pleural space infections in adult and pediatric patients (3 months of age and older) [see Clinical Studies (14.2, 14.5)].
1.3 Treatment of Esophageal Candidiasis
CANCIDAS is indicated for the treatment of esophageal candidiasis in adult and pediatric patients (3 months of age and older) [see Clinical Studies (14.3, 14.5)].
1.4 Treatment of Invasive Aspergillosis in Patients Who Are Refractory to or Intolerant of Other Therapies
CANCIDAS is indicated for the treatment of invasive aspergillosis in adult and pediatric patients (3 months of age and older) who are refractory to or intolerant of other therapies [see Clinical Studies (14.4, 14.5)].
2. Cancidas Dosage and Administration
2.1 Important Administration Instructions for Use in All Patients
Administer CANCIDAS by slow intravenous (IV) infusion over approximately 1 hour. Do not administer CANCIDAS by IV bolus administration.
2.2 Recommended Dosage in Adult Patients [18 years of age and older]
The dosage and duration of CANCIDAS treatment for each indication are as follows:
2.3 Recommended Dosing in Pediatric Patients [3 months to 17 years of age]
For all indications, administer a single 70 mg/m2 loading dose on Day 1, followed by 50 mg/m2 once daily thereafter. The maximum loading dose and the daily maintenance dose should not exceed 70 mg, regardless of the patient's calculated dose. Dosing in pediatric patients (3 months to 17 years of age) should be based on the patient's body surface area (BSA) as calculated by the Mosteller Formula [see References (15)]:

Following calculation of the patient's BSA, the loading dose in milligrams should be calculated as BSA (m2) × 70 mg/m2. The maintenance dose in milligrams should be calculated as BSA (m2) × 50 mg/m2.
Duration of treatment should be individualized to the indication, as described for each indication in adults [see Dosage and Administration (2.2)]. If the 50-mg/m2 daily dose is well tolerated but does not provide an adequate clinical response, the daily dose can be increased to 70 mg/m2 daily (not to exceed 70 mg).
2.4 Dosage Adjustments in Patients with Hepatic Impairment
Adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) do not need a dosage adjustment. For adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9), CANCIDAS 35 mg once daily is recommended based upon pharmacokinetic data [see Clinical Pharmacology (12.3)] with a 70-mg loading dose administered on Day 1 where appropriate. There is no clinical experience in adult patients with severe hepatic impairment (Child-Pugh score greater than 9) and in pediatric patients with any degree of hepatic impairment.
2.7 Drug Incompatibilities
Do not mix or co-infuse CANCIDAS with other medications, as there are no data available on the compatibility of CANCIDAS with other intravenous substances, additives, or medications.
Do not use diluents containing dextrose (α-D-glucose), as CANCIDAS is not stable in diluents containing dextrose.
3. Dosage Forms and Strengths
CANCIDAS 50 mg is a white to off-white lyophilized cake or powder for reconstitution in a single-dose glass vial with a red aluminum band and a plastic cap. CANCIDAS 50-mg vial contains 50 mg of caspofungin equivalent to 55.5 mg of caspofungin acetate.
CANCIDAS 70 mg is a white to off-white lyophilized cake or powder for reconstitution in a single-dose glass vial with a yellow/orange aluminum band and a plastic cap. CANCIDAS 70-mg vial contains 70 mg of caspofungin equivalent to 77.7 mg of caspofungin acetate.
4. Contraindications
CANCIDAS is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to any component of this product [see Adverse Reactions (6)].
5. Warnings and Precautions
5.1 Hypersensitivity
Anaphylaxis and other hypersensitivity reactions have been reported during administration of CANCIDAS.
Possible histamine-mediated adverse reactions, including rash, facial swelling, angioedema, pruritus, sensation of warmth or bronchospasm have been reported.
Cases of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), some with a fatal outcome, have been reported with use of CANCIDAS [see Adverse Reactions (6.2)].
Discontinue CANCIDAS at the first sign or symptom of a hypersensitivity reaction and administer appropriate treatment.
5.2 Hepatic Effects
Laboratory abnormalities in liver function tests have been seen in healthy volunteers and in adult and pediatric patients treated with CANCIDAS. In some adult and pediatric patients with serious underlying conditions who were receiving multiple concomitant medications with CANCIDAS, isolated cases of clinically significant hepatic dysfunction, hepatitis, and hepatic failure have been reported; a causal relationship to CANCIDAS has not been established. Monitor patients who develop abnormal liver function tests during CANCIDAS therapy for evidence of worsening hepatic function and evaluated for risk/benefit of continuing CANCIDAS therapy.
5.3 Elevated Liver Enzymes During Concomitant Use With Cyclosporine
Elevated liver enzymes have occurred in patients receiving CANCIDAS and cyclosporine concomitantly. Only use CANCIDAS and cyclosporine in those patients for whom the potential benefit outweighs the potential risk. Patients who develop abnormal liver enzymes during concomitant therapy should be monitored and the risk/benefit of continuing therapy should be evaluated.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in detail in another section of the labeling:
- Hypersensitivity [see Warnings and Precautions (5.1)]
- Hepatic Effects [see Warnings and Precautions (5.2)]
- Elevated Liver Enzymes During Concomitant Use With Cyclosporine [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of CANCIDAS cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during the post-approval use of CANCIDAS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal disorders: pancreatitis
- Hepatobiliary disorders: hepatic necrosis
- Skin and subcutaneous tissue disorders: erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, and skin exfoliation
- Renal and urinary disorders: clinically significant renal dysfunction
- General disorders and administration site conditions: swelling and peripheral edema
- Laboratory abnormalities: gamma-glutamyltransferase increased
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of CANCIDAS in pediatric patients 3 months to 17 years of age are supported by evidence from adequate and well-controlled studies in adults, pharmacokinetic data in pediatric patients, and additional data from prospective studies in pediatric patients 3 months to 17 years of age for the following indications [see Indications and Usage (1)]:
- Empirical therapy for presumed fungal infections in febrile, neutropenic patients.
- Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis, and pleural space infections.
- Treatment of esophageal candidiasis.
- Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies (e.g., amphotericin B, lipid formulations of amphotericin B, itraconazole).
The efficacy and safety of CANCIDAS has not been adequately studied in prospective clinical trials involving neonates and infants under 3 months of age. Although limited pharmacokinetic data were collected in neonates and infants below 3 months of age, these data are insufficient to establish a safe and effective dose of caspofungin in the treatment of neonatal candidiasis. Invasive candidiasis in neonates has a higher rate of CNS and multi-organ involvement than in older patients; the ability of CANCIDAS to penetrate the blood-brain barrier and to treat patients with meningitis and endocarditis is unknown.
CANCIDAS has not been studied in pediatric patients with endocarditis, osteomyelitis, and meningitis due to Candida. CANCIDAS has also not been studied as initial therapy for invasive aspergillosis in pediatric patients.
In clinical trials, 171 pediatric patients (0 months to 17 years of age), including 18 patients who were less than 3 months of age, were given intravenous CANCIDAS. Pharmacokinetic studies enrolled a total of 66 pediatric patients, and an additional 105 pediatric patients received CANCIDAS in safety and efficacy studies [see Clinical Pharmacology (12.3) and Clinical Studies (14.5)]. The majority of the pediatric patients received CANCIDAS at a once-daily maintenance dose of 50 mg/m2 for a mean duration of 12 days (median 9, range 1-87 days). In all studies, safety was assessed by the investigator throughout study therapy and for 14 days following cessation of study therapy. The most common adverse reactions in pediatric patients treated with CANCIDAS were pyrexia (29%), blood potassium decreased (15%), diarrhea (14%), increased aspartate aminotransferase (12%), rash (12%), increased alanine aminotransferase (11%), hypotension (11%), and chills (11%) [see Adverse Reactions (6.2)].
Postmarketing hepatobiliary adverse reactions have been reported in pediatric patients with serious underlying medical conditions [see Warnings and Precautions (5.3)].
8.5 Geriatric Use
Clinical studies of CANCIDAS did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Although the number of elderly patients was not large enough for a statistical analysis, no overall differences in safety or efficacy were observed between these and younger patients. Plasma concentrations of caspofungin in healthy older men and women (65 years of age and older) were increased slightly (approximately 28% in AUC) compared to young healthy men. A similar effect of age on pharmacokinetics was seen in patients with candidemia or other Candida infections (intra-abdominal abscesses, peritonitis, or pleural space infections). No dose adjustment is recommended for the elderly; however, greater sensitivity of some older individuals cannot be ruled out.
8.6 Patients with Hepatic Impairment
Adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) do not need a dosage adjustment. For adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9), CANCIDAS 35 mg once daily is recommended based upon pharmacokinetic data [see Clinical Pharmacology (12.3)]. However, where recommended, a 70-mg loading dose should still be administered on Day 1 [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)]. There is no clinical experience in adult patients with severe hepatic impairment (Child-Pugh score greater than 9) and in pediatric patients 3 months to 17 years of age with any degree of hepatic impairment.
10. Overdosage
In 6 healthy subjects who received a single 210-mg dose, no significant adverse reactions were reported. Multiple doses above 150 mg daily have not been studied. Caspofungin is not dialyzable.
In clinical trials, one pediatric patient (16 years of age) unintentionally received a single dose of caspofungin of 113 mg (on Day 1), followed by 80 mg daily for an additional 7 days. No clinically significant adverse reactions were reported.
11. Cancidas Description
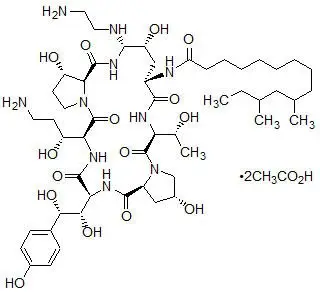
CANCIDAS is a sterile, lyophilized product for intravenous (IV) infusion that contains a semisynthetic lipopeptide (echinocandin) compound synthesized from a fermentation product of Glarea lozoyensis. CANCIDAS is an echinocandin antifungal that inhibits the synthesis of β (1,3)-D-glucan, an integral component of the fungal cell wall.
CANCIDAS (caspofungin acetate) is 1-[(4R,5S)-5-[(2-aminoethyl)amino]-N2-(10,12-dimethyl-1-oxotetradecyl)-4-hydroxy-L-ornithine]-5-[(3R)-3-hydroxy-L-ornithine] pneumocandin B0 diacetate (salt). CANCIDAS 50 mg contains 50 mg of caspofungin equivalent to 55.5 mg of caspofungin acetate. CANCIDAS 50 mg also contains: 39 mg sucrose, 26 mg mannitol, 2 mg glacial acetic acid added as a buffering agent, and sodium hydroxide added as a pH adjuster ingredient. CANCIDAS 70 mg contains 70 mg of caspofungin equivalent to 77.7 mg of caspofungin acetate. CANCIDAS 70 mg also contains 54 mg sucrose, 36 mg mannitol, 2.7 mg glacial acetic acid added as a buffering agent, and sodium hydroxide added as a pH adjuster ingredient.
Caspofungin acetate is a hygroscopic, white to off-white powder. It is freely soluble in water and methanol, and slightly soluble in ethanol. The pH of a saturated aqueous solution of caspofungin acetate is approximately 6.6. The empirical formula is C52H88N10O15∙2C2H4O2 and the formula weight is 1213.42. The structural formula is:
12. Cancidas - Clinical Pharmacology
12.3 Pharmacokinetics
Adult and pediatric pharmacokinetic parameters are presented in Table 8.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of caspofungin.
Caspofungin did not show evidence of mutagenic or genotoxic potential when evaluated in the following in vitro assays: bacterial (Ames) and mammalian cell (V79 Chinese hamster lung fibroblasts) mutagenesis assays, the alkaline elution/rat hepatocyte DNA strand break test, and the chromosome aberration assay in Chinese hamster ovary cells. Caspofungin was not genotoxic when assessed in the mouse bone marrow chromosomal test at doses up to 12.5 mg/kg (equivalent to a human dose of 1 mg/kg based on body surface area comparisons), administered intravenously.
Fertility and reproductive performance were not affected by the intravenous administration of caspofungin to rats at doses up to 5 mg/kg. At 5 mg/kg exposures were similar to those seen in patients treated with the 70-mg dose.
13.2 Animal Toxicology and/or Pharmacology
In one 5-week study in monkeys at doses which produced exposures approximately 4 to 6 times those seen in adult patients treated with a 70-mg dose, scattered small foci of subcapsular necrosis were observed microscopically in the livers of some animals (2/8 monkeys at 5 mg/kg and 4/8 monkeys at 8 mg/kg); however, this histopathological finding was not seen in another study of 27 weeks duration at similar doses.
No treatment-related findings were seen in a 5-week study in infant monkeys at doses which produced exposures approximately 3 times those achieved in pediatric patients receiving a maintenance dose of 50 mg/m2 daily.
14. Clinical Studies
14.1 Empirical Therapy in Febrile, Neutropenic Patients
A double-blind study enrolled 1111 febrile, neutropenic (<500 cells/mm3) patients who were randomized to treatment with daily doses of CANCIDAS (50 mg/day following a 70-mg loading dose on Day 1) or AmBisome (3 mg/kg/day). Patients were stratified based on risk category (high-risk patients had undergone allogeneic stem cell transplantation or had relapsed acute leukemia) and on receipt of prior antifungal prophylaxis. Twenty-four percent of patients were high risk and 56% had received prior antifungal prophylaxis. Patients who remained febrile or clinically deteriorated following 5 days of therapy could receive 70 mg/day of CANCIDAS or 5 mg/kg/day of AmBisome. Treatment was continued to resolution of neutropenia (but not beyond 28 days unless a fungal infection was documented).
An overall favorable response required meeting each of the following criteria: no documented breakthrough fungal infections up to 7 days after completion of treatment, survival for 7 days after completion of study therapy, no discontinuation of the study drug because of drug-related toxicity or lack of efficacy, resolution of fever during the period of neutropenia, and successful treatment of any documented baseline fungal infection.
Based on the composite response rates, CANCIDAS was as effective as AmBisome in empirical therapy of persistent febrile neutropenia (see Table 9).
|
CANCIDAS* |
AmBisome* | % Difference (Confidence Interval)† |
|
|---|---|---|---|
|
|||
| Number of Patients‡ | 556 | 539 | |
| Overall Favorable Response | 190 (33.9%) | 181 (33.7%) | 0.2 (-5.6, 6.0) |
| No documented breakthrough fungal infection | 527 (94.8%) | 515 (95.5%) | -0.8 |
| Survival 7 days after end of treatment | 515 (92.6%) | 481 (89.2%) | 3.4 |
| No discontinuation due to toxicity or lack of efficacy | 499 (89.7%) | 461 (85.5%) | 4.2 |
| Resolution of fever during neutropenia | 229 (41.2%) | 223 (41.4%) | -0.2 |
The rate of successful treatment of documented baseline infections, a component of the primary endpoint, was not statistically different between treatment groups.
The response rates did not differ between treatment groups based on either of the stratification variables: risk category or prior antifungal prophylaxis.
14.2 Candidemia and the Following other Candida Infections: Intra-Abdominal Abscesses, Peritonitis and Pleural Space Infections
In a randomized, double-blind study, patients with a proven diagnosis of invasive candidiasis received daily doses of CANCIDAS (50 mg/day following a 70-mg loading dose on Day 1) or amphotericin B deoxycholate (0.6 to 0.7 mg/kg/day for non-neutropenic patients and 0.7 to 1 mg/kg/day for neutropenic patients). Patients were stratified by both neutropenic status and APACHE II score. Patients with Candida endocarditis, meningitis, or osteomyelitis were excluded from this study.
Patients who met the entry criteria and received one or more doses of IV study therapy were included in the modified intention-to-treat [MITT] analysis of response at the end of IV study therapy. A favorable response at this time point required both symptom/sign resolution/improvement and microbiological clearance of the Candida infection.
Two hundred thirty-nine patients were enrolled. Patient disposition is shown in Table 10.
| CANCIDAS* | Amphotericin B | |
|---|---|---|
|
||
| Randomized patients | 114 | 125 |
| Patients completing study† | 63 (55.3%) | 69 (55.2%) |
| DISCONTINUATIONS OF STUDY† | ||
| All Study Discontinuations | 51 (44.7%) | 56 (44.8%) |
| Study Discontinuations due to clinical adverse events | 39 (34.2%) | 43 (34.4%) |
| Study Discontinuations due to laboratory adverse events | 0 (0%) | 1 (0.8%) |
| DISCONTINUATIONS OF STUDY THERAPY | ||
| All Study Therapy Discontinuations | 48 (42.1%) | 58 (46.4%) |
| Study Therapy Discontinuations due to clinical adverse events | 30 (26.3%) | 37 (29.6%) |
| Study Therapy Discontinuations due to laboratory adverse events | 1 (0.9%) | 7 (5.6%) |
| Study Therapy Discontinuations due to all drug-related‡ adverse events | 3 (2.6%) | 29 (23.2%) |
Of the 239 patients enrolled, 224 met the criteria for inclusion in the MITT population (109 treated with CANCIDAS and 115 treated with amphotericin B). Of these 224 patients, 186 patients had candidemia (92 treated with CANCIDAS and 94 treated with amphotericin B). The majority of the patients with candidemia were non-neutropenic (87%) and had an APACHE II score less than or equal to 20 (77%) in both arms. Most candidemia infections were caused by C. albicans (39%), followed by C. parapsilosis (20%), C. tropicalis (17%), C. glabrata (8%), and C. krusei (3%).
At the end of IV study therapy, CANCIDAS was comparable to amphotericin B in the treatment of candidemia in the MITT population. For the other efficacy time points (Day 10 of IV study therapy, end of all antifungal therapy, 2-week post-therapy follow-up, and 6- to 8-week post-therapy follow-up), CANCIDAS was as effective as amphotericin B.
Outcome, relapse and mortality data are shown in Table 11.
| CANCIDAS* | Amphotericin B | % Difference† after adjusting for strata (Confidence Interval)‡ |
|
|---|---|---|---|
|
|||
| Number of MITT§ patients | 109 | 115 | |
| FAVORABLE OUTCOMES (MITT) AT THE END OF IV STUDY THERAPY | |||
| All MITT patients | 81/109 (74.3%) | 78/115 (67.8%) | 7.5 (-5.4, 20.3) |
| Candidemia | 67/92 (72.8%) | 63/94 (67.0%) | 7.0 (-7.0, 21.1) |
| Neutropenic | 6/14 (43%) | 5/10 (50%) | |
| Non-neutropenic | 61/78 (78%) | 58/84 (69%) | |
| Endophthalmitis | 0/1 | 2/3 | |
| Multiple Sites | 4/5 | 4/4 | |
| Blood / Pleural | 1/1 | 1/1 | |
| Blood / Peritoneal | 1/1 | 1/1 | |
| Blood / Urine | - | 1/1 | |
| Peritoneal / Pleural | 1/2 | - | |
| Abdominal / Peritoneal | - | 1/1 | |
| Subphrenic / Peritoneal | 1/1 | - | |
| DISSEMINATED INFECTIONS, RELAPSES AND MORTALITY | |||
| Disseminated Infections in neutropenic patients | 4/14 (28.6%) | 3/10 (30.0%) | |
| All relapses¶ | 7/81 (8.6%) | 8/78 (10.3%) | |
| Culture-confirmed relapse | 5/81 (6%) | 2/78 (3%) | |
| Overall study# mortality in MITT | 36/109 (33.0%) | 35/115 (30.4%) | |
| Mortality during study therapy | 18/109 (17%) | 13/115 (11%) | |
| Mortality attributed to Candida | 4/109 (4%) | 7/115 (6%) | |
In this study, the efficacy of CANCIDAS in patients with intra-abdominal abscesses, peritonitis and pleural space Candida infections was evaluated in 19 non-neutropenic patients. Two of these patients had concurrent candidemia. Candida was part of a polymicrobial infection that required adjunctive surgical drainage in 11 of these 19 patients. A favorable response was seen in 9 of 9 patients with peritonitis, 3 of 4 with abscesses (liver, parasplenic, and urinary bladder abscesses), 2 of 2 with pleural space infections, 1 of 2 with mixed peritoneal and pleural infection, 1 of 1 with mixed abdominal abscess and peritonitis, and 0 of 1 with Candida pneumonia.
Overall, across all sites of infection included in the study, the efficacy of CANCIDAS was comparable to that of amphotericin B for the primary endpoint.
In this study, the efficacy data for CANCIDAS in neutropenic patients with candidemia were limited. In a separate compassionate use study, 4 patients with hepatosplenic candidiasis received prolonged therapy with CANCIDAS following other long-term antifungal therapy; three of these patients had a favorable response.
In a second randomized, double-blind study, 197 patients with proven invasive candidiasis received CANCIDAS 50 mg/day (following a 70-mg loading dose on Day 1) or CANCIDAS 150 mg/day. The diagnostic criteria, evaluation time points, and efficacy endpoints were similar to those employed in the prior study. Patients with Candida endocarditis, meningitis, or osteomyelitis were excluded. Although this study was designed to compare the safety of the two doses, it was not large enough to detect differences in rare or unexpected adverse events [see Adverse Reactions (6.1)]. The efficacy of CANCIDAS at the 150 mg daily dose was not significantly better than the efficacy of the 50-mg daily dose of CANCIDAS. The efficacy of doses higher than 50 mg daily in the other adult patients for whom CANCIDAS is indicated has not been evaluated.
14.3 Esophageal Candidiasis (and information on oropharyngeal candidiasis)
The safety and efficacy of CANCIDAS in the treatment of esophageal candidiasis was evaluated in one large, controlled, noninferiority, clinical trial and two smaller dose-response studies.
In all 3 studies, patients were required to have symptoms and microbiological documentation of esophageal candidiasis; most patients had advanced AIDS (with CD4 counts <50/mm3).
Of the 166 patients in the large study who had culture-confirmed esophageal candidiasis at baseline, 120 had Candida albicans and 2 had Candida tropicalis as the sole baseline pathogen whereas 44 had mixed baseline cultures containing C. albicans and one or more additional Candida species.
In the large, randomized, double-blind study comparing CANCIDAS 50 mg/day versus intravenous fluconazole 200 mg/day for the treatment of esophageal candidiasis, patients were treated for an average of 9 days (range 7-21 days). Favorable overall response at 5 to 7 days following discontinuation of study therapy required both complete resolution of symptoms and significant endoscopic improvement. The definition of endoscopic response was based on severity of disease at baseline using a 4-grade scale and required at least a two-grade reduction from baseline endoscopic score or reduction to grade 0 for patients with a baseline score of 2 or less.
The proportion of patients with a favorable overall response was comparable for CANCIDAS and fluconazole as shown in Table 12.
| CANCIDAS | Fluconazole | % Difference†
(95% CI) |
|
|---|---|---|---|
|
|||
| Day 5-7 post-treatment | 66/81 (81.5%) | 80/94 (85.1%) | -3.6 (-14.7, 7.5) |
The proportion of patients with a favorable symptom response was also comparable (90.1% and 89.4% for CANCIDAS and fluconazole, respectively). In addition, the proportion of patients with a favorable endoscopic response was comparable (85.2% and 86.2% for CANCIDAS and fluconazole, respectively).
As shown in Table 13, the esophageal candidiasis relapse rates at the Day 14 post-treatment visit were similar for the two groups. At the Day 28 post-treatment visit, the group treated with CANCIDAS had a numerically higher incidence of relapse; however, the difference was not statistically significant.
| CANCIDAS | Fluconazole | % Difference*
(95% CI) |
|
|---|---|---|---|
|
|||
| Day 14 post-treatment | 7/66 (10.6%) | 6/76 (7.9%) | 2.7 (-6.9, 12.3) |
| Day 28 post-treatment | 18/64 (28.1%) | 12/72 (16.7%) | 11.5 (-2.5, 25.4) |
In this trial, which was designed to establish noninferiority of CANCIDAS to fluconazole for the treatment of esophageal candidiasis, 122 (70%) patients also had oropharyngeal candidiasis. A favorable response was defined as complete resolution of all symptoms of oropharyngeal disease and all visible oropharyngeal lesions. The proportion of patients with a favorable oropharyngeal response at the 5- to 7-day post-treatment visit was numerically lower for CANCIDAS; however, the difference was not statistically significant. Oropharyngeal candidiasis relapse rates at Day 14 and Day 28 post-treatment visits were statistically significantly higher for CANCIDAS than for fluconazole. The results are shown in Table 14.
| CANCIDAS | Fluconazole | % Difference*
(95% CI) |
|
|---|---|---|---|
|
|||
| Response Rate Day 5-7 post-treatment | 40/56 (71.4%) | 55/66 (83.3%) | -11.9 (-26.8, 3.0) |
| Relapse Rate Day 14 post-treatment | 17/40 (42.5%) | 7/53 (13.2%) | 29.3 (11.5, 47.1) |
| Relapse Rate Day 28 post-treatment | 23/39 (59.0%) | 18/51 (35.3%) | 23.7 (3.4, 43.9) |
The results from the two smaller dose-ranging studies corroborate the efficacy of CANCIDAS for esophageal candidiasis that was demonstrated in the larger study.
CANCIDAS was associated with favorable outcomes in 7 of 10 esophageal C. albicans infections refractory to at least 200 mg of fluconazole given for 7 days, although the in vitro susceptibility of the infecting isolates to fluconazole was not known.
14.4 Invasive Aspergillosis
Sixty-nine patients between the ages of 18 and 80 with invasive aspergillosis were enrolled in an open-label, noncomparative study to evaluate the safety, tolerability, and efficacy of CANCIDAS. Enrolled patients had previously been refractory to or intolerant of other antifungal therapy(ies). Refractory patients were classified as those who had disease progression or failed to improve despite therapy for at least 7 days with amphotericin B, lipid formulations of amphotericin B, itraconazole, or an investigational azole with reported activity against Aspergillus. Intolerance to previous therapy was defined as a doubling of creatinine (or creatinine ≥2.5 mg/dL while on therapy), other acute reactions, or infusion-related toxicity. To be included in the study, patients with pulmonary disease must have had definite (positive tissue histopathology or positive culture from tissue obtained by an invasive procedure) or probable (positive radiographic or computed tomography evidence with supporting culture from bronchoalveolar lavage or sputum, galactomannan enzyme-linked immunosorbent assay, and/or polymerase chain reaction) invasive aspergillosis. Patients with extrapulmonary disease had to have definite invasive aspergillosis. Patients were administered a single 70-mg loading dose of CANCIDAS and subsequently dosed with 50 mg daily. The mean duration of therapy was 33.7 days, with a range of 1 to 162 days.
An independent expert panel evaluated patient data, including diagnosis of invasive aspergillosis, response and tolerability to previous antifungal therapy, treatment course on CANCIDAS, and clinical outcome.
A favorable response was defined as either complete resolution (complete response) or clinically meaningful improvement (partial response) of all signs and symptoms and attributable radiographic findings. Stable, nonprogressive disease was considered to be an unfavorable response.
Among the 69 patients enrolled in the study, 63 met entry diagnostic criteria and had outcome data; and of these, 52 patients received treatment for greater than 7 days. Fifty-three (84%) were refractory to previous antifungal therapy and 10 (16%) were intolerant. Forty-five patients had pulmonary disease and 18 had extrapulmonary disease. Underlying conditions were hematologic malignancy (N=24), allogeneic bone marrow transplant or stem cell transplant (N=18), organ transplant (N=8), solid tumor (N=3), or other conditions (N=10). All patients in the study received concomitant therapies for their other underlying conditions. Eighteen patients received tacrolimus and CANCIDAS concomitantly, of whom 8 also received mycophenolate mofetil.
Overall, the expert panel determined that 41% (26/63) of patients receiving at least one dose of CANCIDAS had a favorable response. For those patients who received greater than 7 days of therapy with CANCIDAS, 50% (26/52) had a favorable response. The favorable response rates for patients who were either refractory to or intolerant of previous therapies were 36% (19/53) and 70% (7/10), respectively. The response rates among patients with pulmonary disease and extrapulmonary disease were 47% (21/45) and 28% (5/18), respectively. Among patients with extrapulmonary disease, 2 of 8 patients who also had definite, probable, or possible CNS involvement had a favorable response. Two of these 8 patients had progression of disease and manifested CNS involvement while on therapy.
CANCIDAS is effective for the treatment of invasive aspergillosis in patients who are refractory to or intolerant of itraconazole, amphotericin B, and/or lipid formulations of amphotericin B. However, the efficacy of CANCIDAS for initial treatment of invasive aspergillosis has not been evaluated in comparator-controlled clinical studies.
14.5 Pediatric Patients
The safety and efficacy of CANCIDAS were evaluated in pediatric patients 3 months to 17 years of age in two prospective, multicenter clinical trials.
The first study, which enrolled 82 patients between 2 to 17 years of age, was a randomized, double-blind study comparing CANCIDAS (50 mg/m2 IV once daily following a 70-mg/m2 loading dose on Day 1 [not to exceed 70 mg daily]) to AmBisome (3 mg/kg IV daily) in a 2:1 treatment fashion (56 on caspofungin, 26 on AmBisome) as empirical therapy in pediatric patients with persistent fever and neutropenia. The study design and criteria for efficacy assessment were similar to the study in adult patients [see Clinical Studies (14.1)]. Patients were stratified based on risk category (high-risk patients had undergone allogeneic stem cell transplantation or had relapsed acute leukemia). Twenty-seven percent of patients in both treatment groups were high risk. Favorable overall response rates of pediatric patients with persistent fever and neutropenia are presented in Table 15.
|
CANCIDAS |
AmBisome* |
|
|---|---|---|
|
||
| Number of Patients | 56 | 25 |
| Overall Favorable Response | 26/56 (46.4%) | 8/25 (32.0%) |
| High risk | 9/15 (60.0%) | 0/7 (0.0%) |
| Low risk | 17/41 (41.5%) | 8/18 (44.4%) |
The second study was a prospective, open-label, non-comparative study estimating the safety and efficacy of caspofungin in pediatric patients (ages 3 months to 17 years) with candidemia and other Candida infections, esophageal candidiasis, and invasive aspergillosis (as salvage therapy). The study employed diagnostic criteria which were based on established EORTC/MSG criteria of proven or probable infection; these criteria were similar to those criteria employed in the adult studies for these various indications. Similarly, the efficacy time points and endpoints used in this study were similar to those employed in the corresponding adult studies [see Clinical Studies (14.2, 14.3, and 14.4)]. All patients received CANCIDAS at 50 mg/m2 IV once daily following a 70-mg/m2 loading dose on Day 1 (not to exceed 70 mg daily). Among the 49 enrolled patients who received CANCIDAS, 48 were included in the efficacy analysis (one patient excluded due to not having a baseline Aspergillus or Candida infection). Of these 48 patients, 37 had candidemia or other Candida infections, 10 had invasive aspergillosis, and 1 patient had esophageal candidiasis. Most candidemia and other Candida infections were caused by C. albicans (35%), followed by C. parapsilosis (22%), C. tropicalis (14%), and C. glabrata (11%). The favorable response rate, by indication, at the end of caspofungin therapy was as follows: 30/37 (81%) in candidemia or other Candida infections, 5/10 (50%) in invasive aspergillosis, and 1/1 in esophageal candidiasis.
15. References
- Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med 1987 Oct 22;317(17): 1098 (letter).
| CANCIDAS
caspofungin acetate injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CANCIDAS
caspofungin acetate injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |