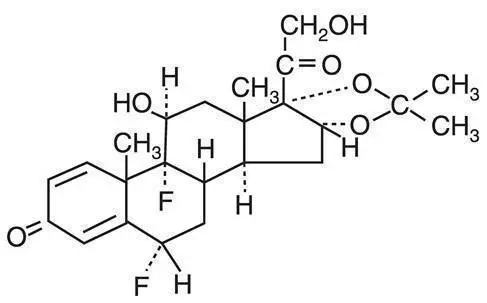
Drug Detail:Capex (Fluocinolone topical [ floo-oh-sin-oh-lone-top-i-kal ])
Drug Class: Topical steroids
Precautions
Pediatric Use:
Safety and effectiveness in children and infants have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression when they are treated with topical corticosteroids. They are therefore also at a greater risk of glucocorticoid insufficiency after withdrawal of treatment and of Cushing’s syndrome while on treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
HPA axis suppression, Cushing’s syndrome and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches and bilateral papilledema.
PATIENT INFORMATION
Capex® (kap-eks) Shampoo
(fluocinolone acetonide)
Topical Shampoo, 0.01%
What is Capex Shampoo?
Capex Shampoo is a prescription medicine used on the scalp to treat a skin condition called seborrheic dermatitis. It is not known if Capex Shampoo is safe and effective in children and infants.
Who should not use Capex Shampoo?
Do not use Capex Shampoo if you are allergic to any of its ingredients. See the end of this leaflet for a complete list of ingredients in Capex Shampoo.
Before using Capex Shampoo, tell your doctor about all of your medical conditions, including if you:
• have a scalp infection. You may need medicine to treat the scalp infection before you use Capex Shampoo.
• are pregnant or plan to become pregnant. It is not known if Capex shampoo can harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
• are breastfeeding or plan to breastfeed. It is not known if Capex Shampoo passes into your breast milk and if it can harm your baby. Talk to your doctor about the best way to feed you baby if you use Capex Shampoo.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your doctor if you take other corticosteroid medicines by mouth or use other products on your skin that contain corticosteroids. Do not use other products containing a corticosteroid medicine with Capex Shampoo without talking to your doctor first.
How should I use Capex Shampoo?
• When you receive your Capex Shampoo, it will already be mixed in a bottle by your pharmacist.
• Use Capex Shampoo exactly as your doctor tells you to use it.
• Your doctor should tell you how much Capex Shampoo to use and where to apply it.
• Capex Shampoo is for use on your scalp only (topical).
• Shake the Capex Shampoo bottle well before using.
• Do not apply more than 1 ounce of Capex Shampoo to your scalp, 1 time each day.
• Work Capex Shampoo into a lather.
• Leave Capex Shampoo on your scalp for at least 5 minutes.
• Rinse your hair and scalp well with water.
• Avoid getting Capex Shampoo in your eyes. If you get Capex Shampoo in your eyes, rinse your eyes well with water.
• Do not bandage or cover your head after using Capex Shampoo unless your doctor tells you to.
• Tell your doctor if you develop irritation where you apply Capex Shampoo, or if your seborrheic dermatitis is not getting better with Capex Shampoo.
What are the possible side effects of Capex Shampoo?
Capex Shampoo can pass through your skin.
The side effects of corticosteroids medicines used on the skin (topical) include:
• dry, itchy, and reddened skin
• burning or stinging at application site
• inflamed hair follicle
• acne
• thinning of skin
• loss of skin color
These are not all the possible side effects of Capex Shampoo. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Capex Shampoo?
• Store Capex Shampoo at room temperature between 68°F to 77°F (20°C to 25°C).
• Keep the Capex Shampoo bottle tightly closed.
• Throw away (discard) your Capex Shampoo bottle after 2 months, even if there is still medicine left in the bottle.
Keep Capex Shampoo and all medicines out of the reach of children.
General information about the safe and effective use of Capex Shampoo.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use Capex Shampoo for a condition for which it was not prescribed. Do not give Capex Shampoo to other people, even if they have the same symptoms you have. It may harm them. You can also ask your doctor or pharmacist for information about Capex Shampoo that is written for health professionals.
What are the ingredients in Capex Shampoo?
Active ingredient: fluocinolone acetonide
Inactive ingredients: aluminum acetate dibasic, benzalkonium chloride solution, boric acid, citric acid anhydrous, cocamido-ether-sulfate complex, cocoamine oxide, dibasic calcium phosphate dihydrate, lauramide DEA, magnesium aluminum silicate, methylparaben, oat flour, propylene glycol, propylparaben, purified water, talc, fragrances, D&C Yellow 10, and FD&C Blue 1 as coloring.
PACKAGE LABEL

ATTN PHARMACIST: Empty contents of enclosed capsule into shampoo bottle.
Rx only
NDC 0299-5500-04
Capex®
(fluocinolone acetonide)
topical shampoo
SHAMPOO (as dispensed)
0.01%
For Topical Use Only
Shake Well Before Use
Discard Contents After Two Months
4 fl oz
GALDERMA
ATTN PHARMACIST: Empty contents of enclosed capsule into shampoo bottle.
Usual Dose: Apply no more than approximately one ounce of shampoo on affected area, let stand for 5 minutes, then rinse thoroughly with water.
See package insert for full prescribing information.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Made in Canada.
All trademarks are the property of their respective owners.
P52307-3
Contains: (As dispensed) Fluocinolone Acetonide (0.01%), dibasic calcium phosphate dihydrate USP, talc USP, in a shampoo base containing aluminum acetate dibasic, benzalkonium chloride solution, boric acid, citric acid anhydrous, cocamido-ether sulfate complex, cocoamine oxide, lauramide DEA, magnesium aluminum silicate, methylparaben, oat flour, propylene glycol, propylparaben, purified water and fragrance with D & C Yellow No. 10 and FD&C Blue No. 1 as coloring.
Storage: Keep tightly closed. Store at controlled room temperature 68° - 77°F (20° - 25°C) with excursions permitted between 59° - 86°F (15° - 30°C)
Contents: 4 fl oz in 6 oz bottle for convenience of Pharmacist.
For External Use Only.
Keep out of reach of children.
Warning: In case of eye contact rinse eye thoroughly with water.
| CAPEX
fluocinolone acetonide kit |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Confab Laboratories | 241754217 | MANUFACTURE(0299-5500) | |