Drug Detail:Carimune (igiv) (Immune globulin (igiv) (intravenous) [ im-myoon-glob-yoo-lin ])
Drug Class: Immune globulins
WARNING: THROMBOSIS, RENAL DYSFUNCTION, or ACUTE RENAL FAILURE
- Thrombosis may occur with immune globulin products1-8, including Carimune NF. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors (see PRECAUTIONS: Thrombosis and Information for Patients).
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients with immune globulin intravenous (IGIV) products9–14, including Carimune NF. Patients predisposed to renal dysfunction include those with any degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. Carimune NF contains sucrose.
- For patients at risk of thrombosis, renal dysfunction or acute renal failure, administer Carimune NF at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity (see DOSAGE AND ADMINISTRATION, and PRECAUTIONS: Thrombosis).
Carimune NF Description
Carimune® NF, Nanofiltered, Immune Globulin Intravenous (Human), is a sterile, highly purified polyvalent antibody product containing in concentrated form all the IgG antibodies which regularly occur in the donor population.15 This immunoglobulin preparation is produced by cold alcohol fractionation from the plasma of US donors. Part of the fractionation may be performed by another US-licensed manufacturer. Carimune® NF is made suitable for intravenous use by treatment at acid pH in the presence of trace amounts of pepsin.16,17 The manufacturing process by which Carimune® NF is prepared from plasma consists of fractionation and purification steps that comprise filtrations in the presence of filter aids. Four of these steps were validated for virus elimination of both enveloped and non-enveloped viruses. Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the vCJD and CJD agents.18 To complement the existing virus elimination / inactivation mechanism in the Carimune® NF manufacturing process, nanofiltration (removing viruses via size-exclusion) was introduced as an additional virus removal step into the manufacturing process.19,20 Nanofiltration is performed prior to the viral inactivation step (pH 4 in presence of pepsin) in order to reduce the potential viral load before inactivation is performed. Treatment with pepsin at pH 4 rapidly inactivates enveloped viruses.21
The Carimune® NF manufacturing process provides a significant virus reduction capacity as shown in in vitro studies. The results, summarized in Table 1, demonstrate virus clearance during Carimune® NF manufacturing using model viruses for lipid enveloped and non-enveloped viruses.
| Virus | HIV | BVDV | PRV | SFV | SV | BEV | |
|---|---|---|---|---|---|---|---|
| HIV: | Human immunodeficiency virus, model for HIV 1 and HIV 2 | ||||||
| BVDV: | Bovine viral diarrhea virus, model for HCV (Hepatitis C virus) | ||||||
| PRV: | Pseudorabies virus, model for large, enveloped DNA viruses (e.g., herpes virus) | ||||||
| SFV: | Semliki Forest virus, model for HCV | ||||||
| SV: | Sindbis virus, model for HCV | ||||||
| BEV: | Bovine enterovirus, model for HAV (Hepatitis A virus) | ||||||
| nt: | not tested | ||||||
| Genome | RNA | RNA | DNA | RNA | RNA | RNA | |
| Envelope | Yes | Yes | Yes | Yes | Yes | No | |
| Size (nm) | 80–100 | 40–60 | 120–200 | 50–70 | 50–70 | 28–30 | |
| Fractionation & Depth filtration | 15.5 | nt | 16.0 | 9.3 | 12.4 | 14.1 | |
| pH 4 / pepsin | ≥ 6.1 | ≥ 4.4 | ≥ 5.3 | ≥ 6.8 | nt | nt | |
| Nanofiltration | ≥ 4.9 | ≥ 4.5 | ≥ 4.4 | nt | ≥ 7.5 | ≥ 5.1 | |
| Overall reduction | ≥ 26 | ≥ 9 | ≥ 25 | ≥ 16 | ≥ 19 | ≥ 19 | |
PRV and the two model viruses for HCV, BVDV and SFV, were inactivated within 1/10, and HIV within 1/2 of the incubation time (pH 4/pepsin treatment) used during production of Carimune® NF.
Several of the individual production steps in the Carimune® NF manufacturing process have been shown to decrease TSE infectivity of an experimental model agent. TSE reduction steps include precipitation (3.5 logs), depth filtrations (7.3 logs), and nanofiltration (4.4 logs). These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed.
The preparation contains at least 96% of IgG and after reconstitution with a neutral unbuffered diluent has a pH of 6.6 ± 0.2. Most of the immunoglobulins are monomeric (7 S) IgG; the remainder consists of dimeric IgG and a small amount of polymeric IgG, traces of IgA and IgM and immunoglobulin fragments.22 The distribution of the IgG subclasses corresponds to that of normal serum.23-26 Final container lyophilized units are prepared so as to contain 3, 6, or 12 g protein with 1.67 g sucrose and less than 20 mg NaCl per gram of protein. The lyophilized preparation contains no preservative and may be reconstituted with sterile water, 5% dextrose or 0.9% saline to a solution with protein concentrations ranging from 3% to 12% (see Table 4). See Table 2 for calculated Carimune® NF osmolality (mOsm/kg) at each protein concentration. The patient's fluid, electrolyte, caloric requirements and renal function should be considered in selecting an appropriate diluent and concentration.
| Concentration | ||||
|---|---|---|---|---|
| Diluent | 3% | 6% | 9% | 12% |
| 0.9% NaCl | 498 | 690 | 882 | 1074 |
| 5% Dextrose | 444 | 636 | 828 | 1020 |
| Sterile Water | 192 | 384 | 576 | 768 |
Contraindications
Carimune® NF is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin. Individuals with IgA deficiency, especially those who have known antibody against IgA, or hypersensitivity to immunoglobulins should only receive Carimune® NF with utmost caution due to the risk of severe immediate hypersensitivity reactions including anaphylaxis.
Warnings
Immune Globulin Intravenous (Human) (IGIV) products have been reported to be associated with renal dysfunction, acute renal failure, osmotic nephrosis, and death.9-14
Patients predisposed to acute renal failure include patients with:
- any degree of pre-existing renal insufficiency
- diabetes mellitus
- age greater than 65
- volume depletion
- sepsis
- paraproteinemia
- patients receiving known nephrotoxic drugs
In such patients, IGIV products should be administered at the minimum concentration available and the minimum rate of infusion practicable. While these reports of renal dysfunction and acute renal failure have been associated with the use of many of the licensed IGIV products, those containing sucrose as a stabilizer accounted for a disproportionate share of the total number. Carimune® NF contains sucrose. See PRECAUTIONS and DOSAGE AND ADMINISTRATION sections for important information intended to reduce the risk of acute renal failure.
IgA deficient patients, especially those with known antibodies against IgA, are at greater risk of developing severe hypersensitivity and anaphylactic reactions.
Because Carimune® NF is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and through the application of viral elimination/reduction steps such as alcohol fractionation in the presence of filter aids, nanofiltration and pH 4/pepsin treatment19-21 (see Table 1). All infections thought by a physician possibly to have been transmitted by Carimune® NF should be reported by lot number, by the physician, or other healthcare provider to CSL Behring Pharmacovigilance at 1-866-915-6958. The physician should discuss the risks and benefits of this product with the patient.
Patients with agamma- or extreme hypogammaglobulinemia who have never before received immunoglobulin substitution treatment or whose time from last treatment is greater than 8 weeks, may be at risk of developing inflammatory reactions on rapid infusion (greater than 2 mg/kg/min) of Carimune® NF. These reactions are manifested by a rise in temperature, chills, nausea, and vomiting. The patient's vital signs should be monitored continuously. The patient should be carefully observed throughout the infusion, since these reactions on rare occasions may lead to shock. Epinephrine and other appropriate resuscitative drugs and equipment should be available for treatment of an acute anaphylactic reaction.
Precautions
Please see DOSAGE AND ADMINISTRATION below, for important information on Carimune® NF compatibility with other medications or fluids. Patients should not be volume depleted prior to the initiation of the infusion of IGIV. Periodic monitoring of renal function tests and urine output is particularly important in patients judged to have a potential increased risk for developing acute renal failure. Renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, should be assessed prior to the initial infusion of Carimune® NF and again at appropriate intervals thereafter. If renal function deteriorates, discontinuation of the product should be considered. For patients judged to be at risk for developing renal dysfunction, Carimune® NF should be infused at a rate less than 2 mg/kg/min.
Information for Patients
- Instruct patients to immediately report symptoms of decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath (which may suggest kidney damage) to their physicians.
- Instruct patients to immediately report symptoms of thrombosis. These symptoms may include: pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body.
Laboratory Tests
IGIV recipients should be monitored for clinical signs and symptoms of hemolysis. IGIV recipients should be monitored for pulmonary adverse reactions. If Transfusion-Related Acute Lung Injury (TRALI) is suspected, appropriate tests should be performed for the presence of anti-neutrophil antibodies in both the product and patient serum. Baseline assessment of blood viscosity should be considered in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies.
Thrombosis
Thrombosis may occur following treatment with immune globulin products1-8, including Carimune NF. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients at risk of thrombosis, administer Carimune NF at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity (see BOXED WARNING, DOSAGE AND ADMINISTRATION, PRECAUTIONS: Information for Patients).
Adverse Reactions/Side Effects
Increases in creatinine and blood urea nitrogen (BUN) have been observed as soon as one to two days following infusion. Progression to oliguria or anuria, requiring dialysis has been observed. Types of severe renal adverse events that have been seen following IGIV therapy include: acute renal failure, acute tubular necrosis, proximal tubular nephropathy and osmotic nephrosis.9-14,64,71–73
Inflammatory adverse reactions have been described in agammaglobulinemic and hypogammaglobulinemic patients who have never received immunoglobulin substitution therapy before or in patients whose time from last treatment is greater than 8 weeks and whose initial infusion rate exceeds 2 mg/kg/min.
This occurs in approximately 10% of such cases. Such reactions may also be observed in some patients during chronic substitution therapy.
Reactions, which may become apparent only 30 minutes to 1 hour after the beginning of the infusion, are as follows: flushing of the face, feelings of tightness in the chest, chills, fever, dizziness, nausea, diaphoresis, and hypotension or hypertension. In such cases, the infusion should be slowed or temporarily stopped until the symptoms subside. The infusion may then be resumed at a lower rate that is comfortable for the patient. If anaphylaxis or other severe reactions occur, the infusion should be stopped immediately.
Arthralgia, myalgia, and transient skin reactions (such as rash, erythema, pruritus, urticaria, eczema or dermatitis) have also been reported.
Immediate anaphylactoid and hypersensitivity reactions due to previous sensitization of the recipient to certain antigens, most commonly IgA, may be observed in exceptional cases, described under CONTRAINDICATIONS.30,31,65 In patients with ITP, who receive higher doses (0.4 g/kg/day or greater), 2.9% of infusions may result in adverse reactions.21 Headache, generally mild, is the most common symptom noted, occurring during or following 2% of infusions. A few cases of usually mild hemolysis have been reported after infusion of intravenous immunoglobulin products.59-61 These were attributed to transferal of blood group (e.g., anti-D) antibodies.
Carimune NF Dosage and Administration
It is generally advisable not to dilute plasma derivatives with other infusable drugs. Carimune® NF should be given by a separate infusion line. No other medications or fluids should be mixed with Carimune® NF preparation.
Carimune® NF should be used with caution in patients with pre-existing renal insufficiency and in patients judged to be at increased risk of developing renal insufficiency (including, but not limited to those with diabetes mellitus, age greater than 65, volume depletion, paraproteinemia, sepsis, and patients receiving known nephrotoxic drugs). In these cases especially it is important to assure that patients are not volume depleted prior to Carimune® NF infusion. No prospective data are presently available to identify a maximum safe dose, concentration, and rate of infusion in patients determined to be at increased risk of acute renal failure. In the absence of prospective data, recommended doses should not be exceeded and the concentration and infusion rate selected should be the minimum practicable. For patients judged to be at risk for developing renal dysfunction, Carimune® NF should be infused at a rate less than 2 mg/kg/min.
For patients judged to be at an increased risk for thrombosis, a maximum infusion rate of less than 2 mg/kg/min for patients is recommended (see PRECAUTIONS: Thrombosis).
If side effects occur, the infusion should be stopped or slowed until the symptoms subside.
Therapy of Idiopathic Thrombocytopenic Purpura (ITP)
Maintenance – Chronic ITP
In adults and children, if after induction therapy the platelet count falls to less than 30,000/µL and/or the patient manifests clinically significant bleeding, 0.4 g/kg of body weight may be given as a single infusion. If an adequate response does not result, the dose can be increased to 0.8–1 g/kg of body weight given as a single infusion.36,69,70
| Concentration (%) | Initial Infusion Rate: 0.5 mg/kg/min | 1 mg/kg/min | 2 mg/kg/min* | Maximum Infusion Rate†: 3 mg/kg/min |
|---|---|---|---|---|
|
||||
| 3% | 0.0167 mL/kg/min | 0.033 mL/kg/min | 0.067 mL/kg/min | 0.10 mL/kg/min |
| 6% | 0.008 mL/kg/min | 0.0167 mL/kg/min | 0.033 mL/kg/min | 0.050 mL/kg/min |
| 9% | 0.006 mL/kg/min | 0.011 mL/kg/min | 0.022 mL/kg/min | 0.033 mL/kg/min |
| 12% | 0.004 mL/kg/min | 0.008 mL/kg/min | 0.016 mL/kg/min | 0.025 mL/kg/min |
Reconstitution
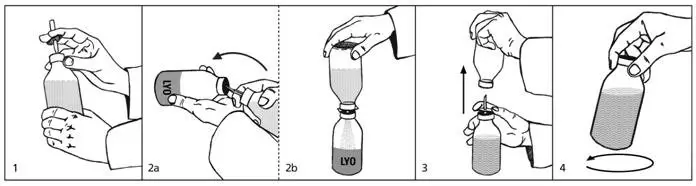
(see also pictures next page)
| 1. | Remove the protective plastic caps from the lyophilisate (LYO) and diluent bottles and disinfect both rubber stoppers with alcohol. Remove the protective cover from one end of the transfer set and insert the exposed needle through the rubber stopper into the bottle containing the diluent (picture 1). |
| 2a. and 2b. | Remove the second protective cover from the other end of the transfer set. Grasp both bottles as shown in picture 2a, quickly plunge the diluent bottle onto the lyophilisate bottle and bring the bottles into an upright position. Only if this is done quickly and the bottles are immediately brought into an upright position can the vacuum in the lyophilisate bottle be maintained, thus speeding up reconstitution and facilitating the transfer. Allow the diluent to flow into the lyophilisate bottle (picture 2b). |
| 3. | Once the appropriate amount of diluent is transferred (see Table 4), lift the diluent bottle off the spike to release the vacuum (picture 3). This will reduce foaming and facilitate dissolution. Remove the spike. |
| 4. | Swirl vigorously but do not shake, otherwise foam will form which is very slow to subside (picture 4). The lyophilisate dissolves within a few minutes. |
To reconstitute Carimune® NF from the individual vial package, or when using other diluents or higher concentrations, Table 4 indicates the volume of sterile diluent required. Observing aseptic technique, this volume should be drawn into a sterile hypodermic syringe and needle. The diluent is then injected into the corresponding Carimune® NF vial size.
| Target Concentration | 6 g Vial | 12 g Vial | |
|---|---|---|---|
|
|||
| 3% | 200 mL | † | |
| 6% | 100 mL | 200 mL | |
| 9% | 66 mL | 132 mL | |
| 12% | 50 mL | 100 mL | |
If large doses of Carimune® NF are to be administered, several reconstituted vials of identical concentration and diluent may be pooled in an empty sterile glass or plastic i.v. infusion container using aseptic technique.
Carimune® NF normally dissolves within a few minutes, though in exceptional cases it may take up to 20 minutes.
DO NOT SHAKE! Excessive shaking will cause foaming.
Any undissolved particles should respond to careful rotation of the bottle. Avoid foaming. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Filtering of Carimune® NF is acceptable but not required. Pore sizes of 15 microns or larger will be less likely to slow infusion, especially with higher Carimune® NF concentrations. Antibacterial filters (0.2 microns) may be used. When reconstitution of Carimune® NF occurs outside of sterile laminar air flow conditions, administration must begin promptly with partially used vials discarded. When reconstitution is carried out in a sterile laminar flow hood using aseptic technique, administration may begin within 24 hours provided the solution has been refrigerated during that time. Do not freeze Carimune® NF solution.
PROCEED WITH INFUSION ONLY IF SOLUTION IS CLEAR AND AT APPROXIMATELY ROOM TEMPERATURE.
| CARIMUNE
NANOFILTERED
human immunoglobulin g injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CARIMUNE
NANOFILTERED
human immunoglobulin g injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CSL Behring AG (481152762) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring AG | 481152762 | MANUFACTURE | |