Drug Detail:Carvykti (Ciltacabtagene autoleucel)
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
CARVYKTI ® (ciltacabtagene autoleucel) suspension for intravenous infusion
Initial U.S. Approval: 2022
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, HLH/MAS and PROLONGED and RECURRENT CYTOPENIA
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients following treatment with CARVYKTI. Do not administer CARVYKTI to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids. ( 2.2, 2.3, 5.1)
- Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), which may be fatal or life-threatening, occurred following treatment with CARVYKTI, including before CRS onset, concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with CARVYKTI. Provide supportive care and/or corticosteroids as needed. ( 2.2, 2.3, 5.2)
- Parkinsonism and Guillain-Barré syndrome and their associated complications resulting in fatal or life-threatening reactions have occurred following treatment with CARVYKTI. ( 5.2)
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS), including fatal and life-threatening reactions, occurred in patients following treatment with CARVYKTI. HLH/MAS can occur with CRS or neurologic toxicities. ( 5.3)
- Prolonged and/or recurrent cytopenias with bleeding and infection and requirement for stem cell transplantation for hematopoietic recovery occurred following treatment with CARVYKTI. ( 5.5)
- CARVYKTI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the CARVYKTI REMS. ( 5.4)
Recent Major Changes
| Warnings and Precautions ( 5.1, 5.2, 5.3, 5.6) | 02/2023 |
Indications and Usage for Carvykti
CARVYKTI is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. ( 1)
Carvykti Dosage and Administration
For autologous use only. For intravenous use only.
- Administer a lymphodepleting regimen of cyclophosphamide and fludarabine before infusion of CARVYKTI. ( 2.2)
- Do NOT use a leukodepleting filter. ( 2.2)
- Verify the patient's identity prior to infusion. ( 2.2)
- Premedicate with acetaminophen and an H1-antihistamine. ( 2.2)
- Avoid prophylactic use of systemic corticosteroids. ( 2.2)
- Confirm availability of tocilizumab prior to infusion. ( 2.2, 5.1)
- Dosing of CARVYKTI is based on the number of chimeric antigen receptor (CAR)-positive viable T cells. ( 2.1)
- Recommended dose range is 0.5–1.0×10 6 CAR-positive viable T cells per kg of body weight, with a maximum dose of 1×10 8 CAR-positive viable T cells per single-dose infusion. ( 2.1)
- Administer CARVYKTI at a REMS-certified healthcare facility. ( 2.2)
Dosage Forms and Strengths
- CARVYKTI is a cell suspension for intravenous infusion. ( 3)
- A single dose of CARVYKTI contains a cell suspension of 0.5–1.0×10 6 CAR-positive viable T cells per kg body weight in one infusion bag. ( 3)
Contraindications
None ( 4)
Warnings and Precautions
- Prolonged and Recurrent Cytopenias: Patients may exhibit ≥Grade 3 cytopenias following CARVYKTI infusion. One or more recurrences of Grade 3 or higher cytopenias may occur after partial or complete recovery of cytopenias. Monitor blood counts prior to and after CARVYKTI infusion. Prolonged neutropenia has been associated with increased risk of infection. ( 5.5)
- Infections: Monitor patients for signs and symptoms of infection; treat appropriately. ( 5.6)
- Hypogammaglobulinemia: Monitor and consider immunoglobulin replacement therapy. ( 5.7)
- Hypersensitivity Reactions: Hypersensitivity reactions have occurred. Monitor for hypersensitivity reactions during infusion. ( 5.8)
- Secondary Malignancies: In the event that a secondary malignancy occurs after treatment with CARVYKTI, contact Janssen Biotech, Inc. at 1-800-526-7736. ( 5.9)
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after receiving CARVYKTI and in the event of any new onset of neurologic toxicities. ( 5.10)
Adverse Reactions/Side Effects
The most common nonlaboratory adverse reactions (incidence greater than 20%) are pyrexia, cytokine release syndrome, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections-pathogen unspecified, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting. The most common laboratory adverse reactions (incidence greater than or equal to 50%) include thrombocytopenia, neutropenia, anemia, aminotransferase elevation and hypoalbuminemia. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2023
Related/similar drugs
Revlimid, Velcade, Pomalyst, Darzalex, Kyprolis, NinlaroFull Prescribing Information
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, HLH/MAS and PROLONGED and RECURRENT CYTOPENIA
Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients following treatment with CARVYKTI. Do not administer CARVYKTI to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)] .
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), which may be fatal or life-threatening, occurred following treatment with CARVYKTI, including before CRS onset, concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with CARVYKTI. Provide supportive care and/or corticosteroids as needed [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.2)] .
Parkinsonism and Guillain-Barré syndrome and their associated complications resulting in fatal or life-threatening reactions have occurred following treatment with CARVYKTI [see Warnings and Precautions (5.2)] .
Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS), including fatal and life-threatening reactions, occurred in patients following treatment with CARVYKTI. HLH/MAS can occur with CRS or neurologic toxicities [see Warnings and Precautions (5.3)].
Prolonged and/or recurrent cytopenias with bleeding and infection and requirement for stem cell transplantation for hematopoietic recovery occurred following treatment with CARVYKTI [see Warnings and Precautions (5.5)] .
CARVYKTI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the CARVYKTI REMS Program [see Warnings and Precautions (5.4)] .
1. Indications and Usage for Carvykti
CARVYKTI is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma, after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
2. Carvykti Dosage and Administration
For autologous use only. For intravenous use only.
2.1 Dose
CARVYKTI is provided as a single dose for infusion containing a suspension of chimeric antigen receptor (CAR)-positive viable T cells in one infusion bag.
The recommended dose range is 0.5–1.0×10 6 CAR-positive viable T cells per kg of body weight, with a maximum dose of 1×10 8 CAR-positive viable T cells per single infusion.
2.2 Administration
CARVYKTI is for autologous use only. The patient's identity must match the patient identifiers on the CARVYKTI cassette and infusion bag. Do not infuse CARVYKTI if the information on the patient-specific labels does not match the intended patient.
2.3 Management of Severe Adverse Reactions
Cytokine Release Syndrome
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)] . Evaluate for and treat other causes of fever, hypoxia and hypotension. Consider laboratory testing to monitor for disseminated intravascular coagulation, hematology parameters, as well as pulmonary, cardiac, renal, and hepatic function. If CRS is suspected, manage according to the recommendations in Table 1.
Patients who experience CRS should be closely monitored for cardiac and other organ function until resolution of symptoms. Consider anti-seizure prophylaxis with levetiracetam in patients who experience CRS.
Patients who experience Grade 2 or higher CRS (e.g., hypotension not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous telemetry and pulse oximetry.
For severe or life-threatening CRS, consider intensive care unit level monitoring and supportive therapy.
For CRS refractory to first line interventions such as tocilizumab or tocilizumab and corticosteroids, consider alternate treatment options (i.e., higher corticosteroid dose, alternative anti-cytokine agents, e.g., anti-IL1 and/or anti-TNFα, anti-T cell therapies). Refractory CRS is characterized by fevers, end-organ toxicity (e.g., hypoxia, hypotension) not improving within 12 hours of first line interventions or development of HLH/MAS.
If concurrent neurologic toxicity is suspected during CRS, administer:
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 1 and 2
- Tocilizumab according to the CRS grade in Table 1
- Anti-seizure medication according to the neurologic toxicity in Table 2
| CRS Grade * | Tocilizumab † | Corticosteroids ‡ |
|---|---|---|
|
||
| Grade 1 | ||
| Temperature ≥38°C § | In patients with:
| N/A |
| Grade 2 | ||
| Symptoms require and respond to moderate intervention.
Temperature ≥38°C § with: Hypotension not requiring vasopressors, and/or, Hypoxia requiring oxygen via canula Þ or blow-by, or, Grade 2 organ toxicity. ¶ | Administer tocilizumab 8 mg/kg IV over 1 hour (not to exceed 800 mg).
Repeat tocilizumab every 8 hours as needed if not responsive to intravenous fluids up to 1 liter or increasing supplemental oxygen. | Consider dexamethasone 10 mg IV every 12–24 hours. |
| If no improvement within 24 hours or rapid progression, repeat tocilizumab and escalate dose and frequency of dexamethasone (20 mg IV every 6 to 12 hours).
If no improvement within 24 hours or continued rapid progression, switch to methylprednisolone 2 mg/kg IV every 12 hours. After 2 doses of tocilizumab, consider alternative anti-cytokine agents. # Do not exceed 3 doses of tocilizumab in 24 hours, or 4 doses in total. |
||
| Grade 3 | ||
| Symptoms require and respond to aggressive intervention.
Temperature ≥38°C § with: Hypotension requiring one vasopressor with or without vasopressin, and/or, Hypoxia requiring oxygen via high-flow nasal canula Þ, facemask, non-rebreather mask, or Venturi mask, or, Grade 3 organ toxicity or Grade 4 transaminitis. | Per Grade 2 | Administer dexamethasone 10 mg IV every 12 hours. |
| If no improvement within 24 hours or rapid progression, repeat tocilizumab and escalate dose and frequency of dexamethasone (20 mg IV every 6 to 12 hours).
If no improvement within 24 hours or continued rapid progression, switch to methylprednisolone 2 mg/kg IV every 12 hours. After 2 doses of tocilizumab, consider alternative anti-cytokine agents. # Do not exceed 3 doses of tocilizumab in 24 hours, or 4 doses in total. |
||
| Grade 4 | ||
| Life-threatening symptoms.
Requirements for ventilator support, continuous veno-venous hemodialysis (CVVHD). Temperature ≥38°C § with: Hypotension requiring multiple vasopressors (excluding vasopressin), and/or, Hypoxia requiring positive pressure (e.g., CPAP, BiPAP, intubation, and mechanical ventilation), or, Grade 4 organ toxicity (excluding transaminitis). | Per Grade 2 | Administer dexamethasone 20 mg IV every 6 hours. |
| After 2 doses of tocilizumab, consider alternative anti-cytokine agents
#. Do not exceed 3 doses of tocilizumab in 24 hours, or 4 doses in total.
If no improvement within 24 hours, consider methylprednisolone (1–2 g IV, repeat every 24 hours if needed; taper as clinically indicated) or other immunosuppressants (e.g. other anti-T cell therapies). |
||
Neurologic Toxicities
Monitor patients for signs and symptoms of neurologic toxicities (ICANS and other neurologic toxicities) (Table 2). Rule out other causes of neurologic signs or symptoms. Provide intensive care and supportive therapy for severe or life-threatening neurologic toxicities. Please see section 5.2 for non ICANS neurologic toxicities. If ICANS is suspected, manage according to the recommendations in Table 2.
If concurrent CRS is suspected during the neurologic toxicity event, administer:
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 1 and 2
- Tocilizumab according to CRS grade in Table 1
- Anti-seizure medication according to neurologic toxicity in Table 2
| ICANS Grade * | Corticosteroids | ||
|---|---|---|---|
| Note: ICANS grade and management is determined by the most severe event (ICE score, level of consciousness, seizure, motor findings, raised ICP/cerebral edema), not attributable to any other cause. | |||
|
|||
| Grade 1
ICE score 7–9 † or depressed level of consciousness: awakens spontaneously. | Consider dexamethasone
‡ 10 mg IV every 12 to 24 hours for 2 to 3 days.
Consider non-sedating, anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
||
| Grade 2
ICE score-3–6 † or depressed level of consciousness: awakens to voice | Administer dexamethasone
‡ 10 mg IV every 12 hours for 2–3 days, or longer for persistent symptoms.
Consider steroid taper if total corticosteroid exposure is greater than 3 days. If no improvement after 24 hours or worsening of neurologic toxicity, increase the dose and/or frequency of dexamethasone up to a maximum of 20 mg IV every 6 hours. Consider non-sedating, anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
||
| Grade 3
ICE score-0–2 † (If ICE score is 0, but the patient is arousable (e.g., awake with global aphasia) and able to perform assessment) or depressed level of consciousness: awakens only to tactile stimulus, or seizures, either:
| Administer dexamethasone
‡ 10 mg-20 mg IV every 6 hours.
If no improvement after 24 hours or worsening of neurologic toxicity, escalate dexamethasone ‡ dose to at least 20 mg IV every 6 hours, OR escalate to high-dose methylprednisolone (1–2 g/day, repeat every 24 hours if needed; taper as clinically indicated) Consider non-sedating, anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis. If cerebral edema is suspected, consider hyperventilation and hyperosmolar therapy. Give high-dose methylprednisolone (1–2 g, repeat every 24 hours if needed; taper as clinically indicated). |
||
| Grade 4
ICE score-0 † (Patient is unarousable and unable to perform ICE assessment) or depressed level of consciousness either:
| Administer dexamethasone
‡ 20 mg IV every 6 hours.
If no improvement after 24 hours or worsening of neurologic toxicity, escalate to high-dose methylprednisolone (1–2 g/day, repeated every 24 hours if needed; taper as clinically indicated). Consider non-sedating, anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis. If raised ICP/cerebral edema is suspected, consider hyperventilation and hyperosmolar therapy. Give high-dose methylprednisolone (1–2 g/day, repeat every 24 hours if needed; taper as clinically indicated), and consider neurology and/or neurosurgery consultation. |
||
3. Dosage Forms and Strengths
CARVYKTI is a cell suspension for intravenous infusion.
A single dose of CARVYKTI contains a cell suspension of 0.5–1.0×10 6 CAR-positive viable T cells per kg body weight in one infusion bag up to a maximum of 1×10 8 CAR-positive viable T cells [see How Supplied/Storage and Handling (16)] .
5. Warnings and Precautions
5.1 Cytokine Release Syndrome
Cytokine release syndrome, including fatal or life-threatening reactions, occurred following treatment with CARVYKTI. CRS occurred in 95% (92/97) of patients receiving ciltacabtagene autoleucel. Grade 3 or higher CRS (2019 ASTCT grade) 1 occurred in 5% (5/97) of patients, with Grade 5 CRS reported in 1 patient. The median time to onset of CRS was 7 days (range: 1 to 12 days). The median duration of CRS was 4 days (range:1 to 40 days) in all but one patient who had a duration of CRS of 97 days with a subsequent fatal outcome. In patients who experienced CRS, the most common manifestations of CRS included pyrexia (100%), hypotension (43%), increased aspartate aminotransferase (AST) (22%), chills (15%), increased alanine aminotransferase (ALT) (14%) and sinus tachycardia (11%). Grade 3 or higher events associated with CRS included increased AST and ALT, hyperbilirubinemia, hypotension, pyrexia, hypoxia, respiratory failure, acute kidney injury, disseminated intravascular coagulation and hemorrhage, HLH/MAS, angina pectoris, supraventricular and ventricular tachycardia, malaise, myalgias, increased-C-reactive protein, ferritin, blood alkaline phosphatase and gamma-glutamyl transferase [see Adverse Reactions (6.1)] .
Identify CRS based on clinical presentation. Evaluate for and treat other causes of fever, hypoxia, and hypotension. CRS has been reported to be associated with findings of HLH/MAS, and the physiology of the syndromes may overlap. HLH/MAS is a potentially life-threatening condition. In patients with progressive symptoms of CRS or refractory CRS despite treatment, evaluate for evidence of HLH/MAS. One patient with CRS and suspected HLH/MAS developed a fatal retroperitoneal hemorrhage in the setting of thrombocytopenia, coagulopathy and anticoagulation in another ongoing study of CARVYKTI. Please see Section 5.3; Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS).
Sixty-nine of 97 (71%) patients received tocilizumab and/or a corticosteroid for CRS after infusion of ciltacabtagene autoleucel. Forty-four (45%) patients received tocilizumab without corticosteroids, of whom 33 (34%) received a single dose and 11 (11%) received more than 1 dose; 24 patients (25%) received tocilizumab and a corticosteroid, and one patient (1%) received only corticosteroids.
Ensure that a minimum of two doses of tocilizumab are available prior to infusion of CARVYKTI.
Monitor patients at least daily for 10 days following CARVYKTI infusion at a REMS-certified healthcare facility for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for at least 4 weeks after infusion. At the first sign of CRS, immediately institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids, as indicated in Table 1 [see Dosing and Administration (2.3)] .
Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling information (17)].
5.2 Neurologic Toxicities
Neurologic toxicities, which may be severe, life-threatening or fatal, occurred following treatment with CARVYKTI. Neurologic toxicities included ICANS, neurologic toxicity with signs and symptoms of parkinsonism, Guillain-Barré Syndrome, immune mediated myelitis, peripheral neuropathies and cranial nerve palsies. Counsel patients on the signs and symptoms of these neurologic toxicities, and on the delayed nature of onset of some of these toxicities. Instruct patients to seek immediate medical attention for further assessment and management if signs or symptoms of any of these neurologic toxicities occur at any time [see Patient Counseling Information (17)] .
Overall, one or more subtypes of neurologic toxicity 2 described below occurred following ciltacabtagene autoleucel infusion in 26% (25/97) of patients of which 11% (11/97) of patients experienced Grade 3 or higher events. These subtypes of neurologic toxicities were also observed in 2 ongoing studies [see Adverse Reactions (6.1)].
5.3 Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS)
Fatal HLH occurred in one patient (1%), 99 days after ciltacabtagene autoleucel infusion. The HLH event was preceded by prolonged CRS lasting 97 days.
The manifestations of HLH/MAS include hypotension, hypoxia with diffuse alveolar damage, coagulopathy, cytopenia and multi-organ dysfunction, including renal dysfunction.
One patient with grade 4 HLH/MAS developed fatal intracerebral and gastrointestinal hemorrhage in the setting of coagulopathy and thrombocytopenia 12 days after treatment in another ongoing study of CARVYKTI [see Warnings and Precautions (5.1)] . Patients who develop HLH/MAS have an increased risk of severe bleeding. Monitor hematological parameters in patients with HLH/MAS and transfuse per institutional guidelines.
HLH is a life-threatening condition with a high mortality rate if not recognized and treated early. Treatment of HLH/MAS should be administered per institutional standards.
5.4 CARVYKTI REMS
Because of the risk of CRS and neurologic toxicities, CARVYKTI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the CARVYKTI REMS [see Boxed Warning, Warnings and Precautions (5.1, 5.2)] . The required components of the CARVYKTI REMS are:
- Healthcare facilities that dispense and administer CARVYKTI must be enrolled and comply with the REMS requirements.
- Certified healthcare facilities must have on-site, immediate access to tocilizumab.
- Ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after CARVYKTI infusion, if needed for treatment of CRS.
- Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense or administer CARVYKTI are trained in the management of CRS and neurologic toxicities.
Further information is available at www.carvyktirems.com or 1-844-672-0067.
5.5 Prolonged and Recurrent Cytopenias
Patients may exhibit prolonged and recurrent cytopenias following lymphodepleting chemotherapy and CARVYKTI infusion. In Study CARTITUDE-1 (N=97), 30% (29/97) of patients experienced prolonged Grade 3 or 4 neutropenia and 41% (40/97) of patients experienced prolonged Grade 3 or 4 thrombocytopenia that had not resolved by Day 30 following ciltacabtagene autoleucel infusion. In 31% (29/95) of patients who recovered from Grade 3 or 4 neutropenia after 1 month, the median time to recovery from ciltacabtagene autoleucel infusion was 1.8 months (range: 1.0 to 3.7 months). In 52% (32/61) of patients who recovered from Grade 3 or 4 thrombocytopenia after 1 month, the median time to recovery from ciltacabtagene autoleucel infusion was 1.9 months (range: 1.1 to 8.5 months).
One patient underwent autologous stem cell therapy for hematopoietic reconstitution due to prolonged thrombocytopenia.
Recurrent Grade 3 or 4 neutropenia, thrombocytopenia, lymphopenia, and anemia were seen in 63% (61/97), 18% (17/97), 60% (58/97), and 37% (36/97) after recovery from initial Grade 3 or 4 cytopenia following ciltacabtagene autoleucel infusion. After Day 60 following ciltacabtagene autoleucel, 31%, 12%, and 6% of patients had a recurrence of Grade 3 or higher lymphopenia, neutropenia, and thrombocytopenia, respectively, after initial recovery of their Grade 3 or 4 cytopenia [see Adverse Reactions (6.1)] . Eighty-seven percent (84/97) of patients had one, two or three or more recurrences of Grade 3 or 4 cytopenias after initial recovery of Grade 3 or 4 cytopenia. Six and 11 patients had Grade 3 or 4 neutropenia and thrombocytopenia respectively at the time of death.
Monitor blood counts prior to and after CARVYKTI infusion. Manage cytopenias with growth factors and blood product transfusion support according to local institutional guidelines.
5.6 Infections
CARVYKTI should not be administered to patients with active infection or inflammatory disorders. Severe, life-threatening, or fatal infections, occurred in patients after CARVYKTI infusion [see Adverse Reactions (6.1)] .
Infections (all grades) occurred in 57 (59%) patients. Grade 3 or 4 infections occurred in 23% (22/97) of patients; Grade 3 or 4 infections with an unspecified pathogen occurred in 17%, viral infections in 7%, bacterial infections in 1%, and fungal infections in 1% of patients. Overall, 4 patients had Grade 5 infections: lung abscess (n=1), sepsis (n=2) and pneumonia (n=1).
Grade 5 infections reported in other studies with CARVYKTI include bronchopulmonary aspergillosis, pneumocystis jirovecii pneumonia, and CMV colitis (with HSV-1 hepatitis). Another patient developed mycotic aneurysm due to cerebral aspergillosis and died of subarachnoid hemorrhage.
Monitor patients for signs and symptoms of infection before and after CARVYKTI infusion and treat patients appropriately. Administer prophylactic, pre-emptive and/or therapeutic antimicrobials according to the standard institutional guidelines. Febrile neutropenia was observed in 10% of patients after ciltacabtagene autoleucel infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids and other supportive care, as medically indicated.
In a randomized controlled study of relapsed or refractory multiple myeloma (CARTITUDE- 4), patients treated with ciltacabtagene autoleucel had an increased rate of fatal COVID-19 infections compared to the standard therapy arm. Counsel patients on the importance of prevention measures. Follow institutional guidelines for the vaccination and management of immunocompromised patients with COVID 19.
5.7 Hypogammaglobulinemia
Hypogammaglobulinemia can occur in patients receiving treatment with CARVYKTI. Hypogammaglobulinemia was reported as an adverse event in 12% (12/97) of patients; laboratory IgG levels fell below 500 mg/dL after infusion in 92% (89/97) of patients treated with ciltacabtagene autoleucel. Hypogammaglobulinemia either as an adverse reaction or a laboratory IgG level below 500 mg/dL, after infusion occurred in 94% (91/97) of patients treated with ciltacabtagene autoleucel. Thirty-eight percent of patients received intravenous immunoglobulin (IVIG) post ciltacabtagene autoleucel for either an adverse reaction or prophylaxis.
Monitor immunoglobulin levels after treatment with CARVYKTI and administer IVIG for IgG <400 mg/dL. Manage per local institutional guidelines, including infection precautions and antibiotic or antiviral prophylaxis.
5.8 Hypersensitivity Reactions
Hypersensitivity reactions have occurred in 5% (5/97) of patients following ciltacabtagene autoleucel infusion. All reactions were Grade 1 and symptoms included flushing (n=4), chest discomfort (n=2), tachycardia (n=1), wheezing (n=1), tremor (n=1), and burning sensation (n=1). Serious hypersensitivity reactions, including anaphylaxis, may be due to the dimethyl sulfoxide (DMSO) in CARVYKTI. Patients should be carefully monitored for 2 hours after infusion for signs and symptoms of severe reaction. Treat promptly and manage patients appropriately according to the severity of the hypersensitivity reaction.
5.9 Secondary Malignancies
Patients treated with CARVYKTI may develop secondary malignancies. Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Janssen Biotech, Inc. at 1-800-526-7736 for reporting and to obtain instructions on collection of patient samples for testing of secondary malignancy of T cell origin.
5.10 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including altered mental status, seizures, neurocognitive decline or neuropathy, patients receiving CARVYKTI are at risk for altered or decreased consciousness or coordination in the 8 weeks following CARVYKTI infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery during this initial period, and in the event of new onset of any neurologic toxicities.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are also described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)] .
- Neurologic Toxicities [see Warnings and Precautions (5.2)] .
- Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS) [see Warnings and Precautions (5.3)] .
- Prolonged and Recurrent Cytopenias [see Warnings and Precautions (5.5)] .
- Infections [see Warnings and Precautions (5.6)] .
- Hypogammaglobulinemia [see Warnings and Precautions (5.7)] .
- Hypersensitivity Reactions [see Warnings and Precautions (5.8)] .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflect the exposure of 97 adult patients with relapsed/refractory multiple myeloma in the CARTITUDE-1 study (USA cohort) to ciltacabtagene autoleucel and includes 17 patients (18%) with manufacturing failures either because they received ciltacabtagene autoleucel that did not meet product release specifications or there were insufficient data to confirm product release specifications for CARVYKTI. Patients received ciltacabtagene autoleucel across a dose range of 0.51 to 0.95×10 6 CAR-positive viable T cells/kg body weight [see Clinical Studies (14)] . Patients with a history of CNS disease (such as seizure or cerebrovascular ischemia) or requiring ongoing treatment with chronic immunosuppression were excluded. The median duration of follow-up was 18 months. The median age of the study population was 61 years (range: 43 to 78 years); 36% were 65 years or older, and 59% were men. The Eastern Cooperative Oncology Group (ECOG) performance status at baseline was 0 in 40%, 1 in 56%, and 2 in 4% of patients. Three of the patients treated with ciltacabtagene autoleucel had a creatinine clearance of <45 mL/min at baseline. For the details about the study population, see Clinical Studies (14).
The safety data in the Warnings and Precautions section also reflects exposure to ciltacabtagene autoleucel in two ongoing, open-label studies, including patients with previously untreated and relapsed/refractory multiple myeloma in a non-randomized, multi-cohort study (CARTITUDE-2) and patients with relapsed/refractory multiple myeloma in a randomized controlled study (CARTITUDE-4).
The most common (greater or equal to 10%) Grade 3 or 4 nonlaboratory adverse reactions were infections-pathogen unspecified (17%), pneumonia (11%), febrile neutropenia (10%), and hypotension (10%).
The most common nonlaboratory adverse reactions (incidence greater than or equal to 20%) included pyrexia, cytokine release syndrome, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infections of unspecified pathogen, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
Serious adverse reactions occurred in 55% of patients. The most common non-laboratory (greater than or equal to 5%) serious adverse reactions included CRS (21%), sepsis (7%), encephalopathy (10%), and pneumonia (7%). Fatal adverse reactions occurred in 9% of patients.
Table 3 summarizes the adverse reactions that occurred in at least 10% of patients treated with ciltacabtagene autoleucel.
Table 4 describes the most common Grade 3 or 4 laboratory abnormalities.
| System Organ Class (SOC) Preferred term | Any Grade (%) | Grade 3 or higher (%) |
|---|---|---|
| Adverse reactions are reported using MedDRA version 23.0 | ||
|
||
| Blood and lymphatic system disorders | ||
| Coagulopathy * | 22 | 2.1 |
| Febrile Neutropenia | 10 | 10 |
| Cardiac disorders | ||
| Tachycardia † | 27 | 1 |
| Gastrointestinal disorders | ||
| Diarrhea ‡ | 33 | 1 |
| Nausea | 31 | 1 |
| Constipation | 22 | 0 |
| Vomiting | 20 | 0 |
| General disorders and administrative site conditions | ||
| Pyrexia | 96 | 5 |
| Fatigue § | 47 | 7 |
| Chills | 33 | 0 |
| Edema ¶ | 23 | 0 |
| Immune system disorders | ||
| Cytokine release syndrome # | 95 | 5 |
| Hypogammaglobulinemia Þ | 94 | 2 |
| Infections and infestations ß | ||
| Infections-pathogen unspecified à | 41 | 17 |
| Upper respiratory tract infection è | 28 | 3 |
| Viral infections ð | 23 | 7 |
| Pneumonia ø | 12 | 11 |
| Sepsis ý | 10 | 7 |
| Bacterial infections £ | 10 | 3 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 29 | 1 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain ¥ | 48 | 2 |
| Nervous system disorders | ||
| Encephalopathy Π| 30 | 6 |
| Headache | 27 | 0 |
| Dizziness œ | 23 | 1 |
| Motor dysfunction Ɖ | 16 | 3 |
| Psychiatric disorders | ||
| Insomnia | 13 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough A | 39 | 0 |
| Dyspnea B | 23 | 3 |
| Nasal congestion | 15 | 0 |
| Hypoxia | 12 | 4 |
| Vascular disorders | ||
| Hypotension C | 51 | 10 |
| Hypertension | 19 | 6 |
| Hemorrhage D | 15 | 4 |
Other clinically important adverse reactions that occurred in less than 10% of patients treated with ciltacabtagene autoleucel include the following:
- Cardiac disorders: cardiac arrhythmias 1 (8%), chest pain 2 (7%)
- Eye disorders: diplopia (1%)
- Gastrointestinal disorders: dysphagia (1%)
- Immune system disorders: hemophagocytic lymphohistiocytosis (1%), hypersensitivity reaction (5%)
- Infections and Infestations: urinary tract infection 3 (4.1%)
- Injury, Poisoning and Procedural complications: fall (3.1%)
- Metabolism and Nutrition Disorders: tumor lysis syndrome (1%)
- Musculoskeletal and Connective tissue disorders: posture abnormal (1%)
- Nervous system disorders: aphasia 4 (8%), ataxia 5 (8%), tremor (6%), peripheral neuropathy (6%), parkinsonism (4.1%), micrographia (4.1%), dysgraphia (3.1%), reduced facial expression (3.1%), cranial nerve palsies (3.1%), bradykinesia (2.1%), paresis 6 (1%), cogwheel rigidity (1%), cerebrovascular accident (1%), seizure (1%), low speech (1%), nystagmus (1%)
- Psychiatric disorders: delirium 7 (5%) depression 8 (4.1%), psychomotor retardation (1%)
- Renal and urinary disorders: renal failure 9 (7%)
- Skin and subcutaneous tissues: rash 10 (8%)
- Vascular Disorders: thrombosis 11 (5%)
- 1
- Cardiac arrhythmias includes atrial fibrillation, atrial flutter, supraventricular tachycardia, ventricular extrasystoles, ventricular tachycardia.
- 2
- Chest pain includes Angina pectoris, Chest discomfort, and Chest pain.
- 3
- Urinary tract infection includes Urinary tract infection, and Urinary tract infection viral.
- 4
- Aphasia includes Aphasia, Dysarthria, and Speech disorder.
- 5
- Ataxia includes Ataxia, Balance disorder, and Gait disturbance.
- 6
- Paresis includes Facial paralysis, and Peroneal nerve palsy.
- 7
- Delirium includes Agitation, Hallucination, Irritability, Personality change, and Restlessness.
- 8
- Depression includes Depression, and Flat affect.
- 9
- Renal failure includes Acute kidney injury, Blood creatinine increased, Chronic kidney disease, and Renal impairment.
- 10
- Rash includes Erythema, Rash, Rash maculo-papular, and Rash pustular.
- 11
- Thrombosis includes Deep vein thrombosis, and Device related thrombosis.
Laboratory Abnormalities
Table 4 presents the most common Grade 3 or 4 laboratory abnormalities based on laboratory data, occurring in at least 10% of patients.
| Laboratory Abnormality | Grade 3 or 4 (%) |
|---|---|
| Laboratory abnormalities graded using NCI Common Terminology Criteria for Adverse Events version 5.0. Laboratory abnormalities are sorted by decreasing frequency in the Grade column. | |
| Lymphopenia | 99 |
| Neutropenia | 98 |
| White blood cell decreased | 98 |
| Anemia | 72 |
| Thrombocytopenia | 63 |
| Aspartate aminotransferase increased | 21 |
Other clinically important Grade 3 or 4 laboratory abnormalities (based on laboratory data) that occurred in less than 10% of patients treated with ciltacabtagene autoleucel include the following: fibrinogen decreased, hypoalbuminemia, alanine aminotransferase increased, hyponatremia, hypocalcemia, gamma glutamyl transferase increased, alkaline phosphatase increased, hypokalemia, blood bilirubin increased.
6.2 Immunogenicity
The immunogenicity of CARVYKTI has been evaluated using a validated assay for the detection of binding antibodies against the extracellular portion of the anti-BCMA CAR pre-dose, and at multiple timepoints post-infusion. In Study CARTITUDE-1, 19 of 97 (19.6%) patients were positive for anti-product antibodies.
There was no clear evidence that the observed anti-product antibodies impact CARVYKTI kinetics of initial expansion and persistence, efficacy, or safety.
7. Drug Interactions
HIV and the lentivirus used to make CARVYKTI have limited, short spans of identical genetic material (RNA). Therefore, some commercial HIV nucleic acid tests (NATs) may yield false-positive results in patients who have received CARVYKTI.
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of CARVYKTI in pediatric patients have not been established.
8.5 Geriatric Use
Of the 97 patients in Study CARTITUDE-1 that received ciltacabtagene autoleucel, 28% were 65 to 75 years of age, and 8% were 75 years of age or older. CARTITUDE-1 did not include sufficient numbers of patients aged 65 and older to determine whether the effectiveness differs compared with that of younger patients. In 62 patients less than 65 years of age, all grade and Grade 3 and higher neurologic toxicities occurred in 19% (12/62) and 6% (4/62) respectively. Of the 35 patients ≥65 years of age, all grade and Grade 3 and higher neurologic toxicities occurred in 37% (13/35) and 20% (7/35) respectively.
11. Carvykti Description
CARVYKTI ® (ciltacabtagene autoleucel) is a BCMA-directed genetically modified autologous T cell immunotherapy. CARVYKTI is prepared from the patient's peripheral blood mononuclear cells, which are obtained via a standard leukapheresis procedure. The mononuclear cells are enriched for T cells and genetically modified ex vivo by transduction with a replication-incompetent lentiviral vector to express a chimeric antigen receptor (CAR) comprising an anti-BCMA targeting domain, which consists of two single-domain antibodies linked to a 4-1BB costimulatory domain and a CD3-zeta signaling domain.
The transduced anti-BCMA CAR T cells are expanded in cell culture, washed, formulated into a suspension and cryopreserved. The product must pass a sterility test before release for shipping as a frozen suspension in a patient-specific infusion bag. The product is thawed and then infused back into the patient, where the anti-BCMA CAR T cells can recognize and eliminate BCMA-expressing target cells. [see Dosage and Administration (2.2), How Supplied/Storage and Handling (16)].
In addition to T cells, CARVYKTI may contain Natural Killer (NK) cells. The formulation contains 5% dimethyl sulfoxide (DMSO).
12. Carvykti - Clinical Pharmacology
12.1 Mechanism of Action
CARVYKTI is a BCMA-directed, genetically modified autologous T cell immunotherapy, which involves reprogramming a patient's own T cells with a transgene encoding a chimeric antigen receptor (CAR) that identifies and eliminates cells that express BCMA. The CARVYKTI CAR protein features two BCMA-targeting single-domain antibodies designed to confer high avidity against human BCMA, a 4-1BB co-stimulatory domain and a CD3-zeta (CD3ζ) signaling cytoplasmic domain. Upon binding to BCMA-expressing cells, the CAR promotes T cell activation, expansion, and elimination of target cells.
12.2 Pharmacodynamics
After a single infusion of ciltacabtagene autoleucel, expansion of CAR-positive T cells coincided with decreases of serum soluble BCMA, serum M-protein, and/or free light chains. Across all patients, levels of IL-6, IL-10, IFN-γ and IL-2 receptor alpha increased post-infusion and peaked at Days 7–14. The serum levels of all cytokines generally returned to baseline levels within 2–3 months post-infusion.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of ciltacabtagene autoleucel was assessed in 97 patients with multiple myeloma receiving a single infusion at the median dose of 0.71×10 6 CAR positive viable T cells/kg (range: 0.51×10 6 to 0.95×10 6 cells/kg).
Following a single infusion, ciltacabtagene autoleucel exhibited an initial expansion phase followed by a rapid decline, and then a slower decline. However, high inter-individual variability was observed.
| Parameter | Summary Statistics | N=97 |
|---|---|---|
| C max (copies/µg genomic DNA) | Median (range), n | 47806 (7189 – 115234), 97 |
| t max (day) | Median (range), n | 12.7 (8.7 – 329.8), 97 |
| AUC 0–28d (copies*day/µg genomic DNA) | Median (range), n | 371569 (58691 – 2024126), 97 |
| t 1/2 (day) | Median (range), n | 15.3 (3.0 – 95.4), 42 |
After the cell expansion, the persistence phase of ciltacabtagene autoleucel was observed for all patients. At the time of analysis (n=65), the median time for CAR transgene levels in peripheral blood to return to the pre-dose baseline level was approximately 100 days (range: 28 to 365 days) post-infusion.
Detectable ciltacabtagene autoleucel exposures in bone marrow indicate a distribution of ciltacabtagene autoleucel from systemic circulation to bone marrow. Similar to blood transgene levels, bone marrow transgene levels declined over time and exhibited high inter-individual variability.
Some patients required tocilizumab, corticosteroids, and anakinra for the management of CRS. Ciltacabtagene autoleucel continues to expand and persist following administration of tocilizumab, corticosteroids, and anakinra. Ciltacabtagene autoleucel median C max and AUC 0–28d in patients treated with tocilizumab (n=68) for CRS were 168% and 209% of those in patients (n=29) who did not receive tocilizumab for CRS, respectively. The median C max and AUC 0–28d of ciltacabtagene autoleucel in patients who received corticosteroids (n=21) for CRS were 186% and 307% of those in patients who did not receive corticosteroids (n=76) for CRS, respectively. In addition, the median C max and AUC 0–28d of ciltacabtagene autoleucel in patients who received anakinra (n=18) for CRS were 139% and 232% of those in patients who did not receive anakinra (n=79) for CRS, respectively.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No genotoxicity or carcinogenicity studies have been performed with CARVYKTI as they were not indicated. In vitro studies with CARVYKTI manufactured from healthy donors and patients with multiple myeloma showed no evidence of cytokine independent growth and no preferential integration near genes associated with oncogenic transformation.
No studies have been conducted to evaluate the effects of CARVYKTI on fertility.
14. Clinical Studies
The efficacy of ciltacabtagene autoleucel was evaluated in CARTITUDE-1 (NCT03548207), an open-label, single-arm, multicenter trial in adult patients with relapsed or refractory multiple myeloma, who previously received at least 3 prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody [see Adverse Reactions (6.1)] .
Patients with known active or prior history of significant central nervous system (CNS) disease, including CNS multiple myeloma, plasma cell leukemia, allogeneic stem cell transplant within 6 months before apheresis or ongoing treatment with immunosuppressants, creatinine clearance <40 mL/min, absolute lymphocyte concentration <300/µL, absolute neutrophil count <750 cells/mm 3, platelet count <50,000/mm 3, hepatic transaminases >3 times the upper limit of normal, cardiac ejection fraction <45%, or with active serious infection were excluded from the trial.
Of the 113 patients who underwent leukapheresis, 16 patients did not receive ciltacabtagene autoleucel due to progressive disease (n=2), death (n=9), or withdrawal from study (n=5). There were 97 patients in the efficacy evaluable population who received ciltacabtagene autoleucel, including 17 patients (18%) with manufacturing failures either because they received ciltacabtagene autoleucel that did not meet product release specifications for CARVYKTI or received ciltacabtagene autoleucel for which there were insufficient data to confirm product release specifications for CARVYKTI.
Of the 97 efficacy-evaluable patients, the median age was 61 years (range: 43 to 78 years), 59% were male, 71% were white, and 18% were black. Most patients (86%) were International Staging System (ISS) Stage I or II. Of the 91 patients for whom baseline cytogenetic data were available, high-risk cytogenetics (presence of t(4:14), t(14:16), or 17p13 del) were present in 24% of patients. Thirteen percent of the patients had extramedullary disease.
The median number of prior lines of therapy was 6 (range: 3 to 18), with 82% of patients receiving 4 or more prior lines of therapy, 90% of patients had received prior autologous stem cell transplantation (ASCT) and 8% of patients received an allogeneic transplant. Ninety-nine percent of patients were refractory to their last line of prior therapy, and 88% were refractory to a proteasome inhibitor (PI), immunomodulatory agent, and anti-CD38 antibody.
Most patients (75%) treated with ciltacabtagene autoleucel received bridging therapy for control of their multiple myeloma during the manufacturing process. The median time from leukapheresis to product availability was 32 days (range: 27 to 66 days).
The most commonly used agents as bridging therapies (≥20% of patients) included dexamethasone: 62 patients (64%), bortezomib: 26 patients (27%), cyclophosphamide: 22 patients (23%), and pomalidomide: 21 patients (22%).
Efficacy was established on the basis of overall response rate, complete response rate and duration of response as assessed by the Independent Review Committee (IRC) using International Myeloma Working Group (IMWG) criteria (see Table 6). The median time to first response was 1 month (range: 0.9 to 10.7 months).
| Ciltacabtagene autoleucel treated
(N=97) |
|
|---|---|
| Notes: Based on a median duration of follow-up of 18 months.
CI=confidence interval; IRC=Independent Review Committee; IMWG=International Myeloma Working Group; NE=not estimable. |
|
|
|
| Overall Response Rate (sCR * + VGPR + PR) n (%) | 95 (97.9) |
| 95% CI (%) | (92.7, 99.7) |
| Stringent complete response (sCR) * n (%) | 76 (78.4) |
| 95% CI † (%) | (68.8, 86.1) |
| Very good partial response (VGPR) n (%) | 16 (16.5) |
| 95% CI † (%) | (9.7, 25.4) |
| Partial response (PR) n (%) | 3 (3.1) |
| 95% CI † (%) | (0.6, 8.8) |
| Duration of Response (DOR) | |
| Number of responders
DOR (Months):Median (95% CI) ‡ | 95
21.8 (21.8, NE) |
| Number of responders with sCR
*
DOR if best response is sCR * (Months):Median (95% CI) ‡ | 76
NE (21.8, NE) |
| Number of responders with VGPR or better
DOR if best response is VGPR or better (Months):Median (95% CI) ‡ | 92
21.8 (21.8, NE) |
The IRC assessed overall response in the 113 patients that underwent leukapheresis was 84% (95% CI: 76, 90) with stringent CR rate of 67% (95% CI: 58, 76), VGPR rate of 14% (95% CI: 8, 22) and PR rate of 3% (95% CI: 1, 8).
15. References
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019; 25: 625–638.
- National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v 5.0; 2017.
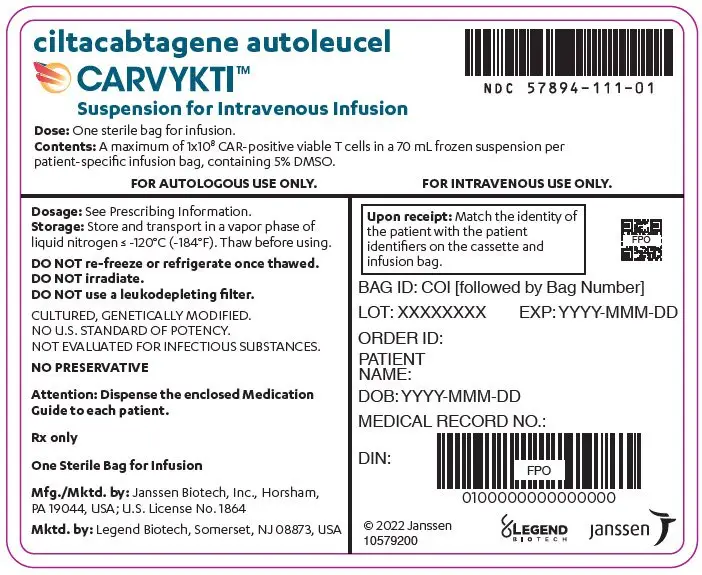
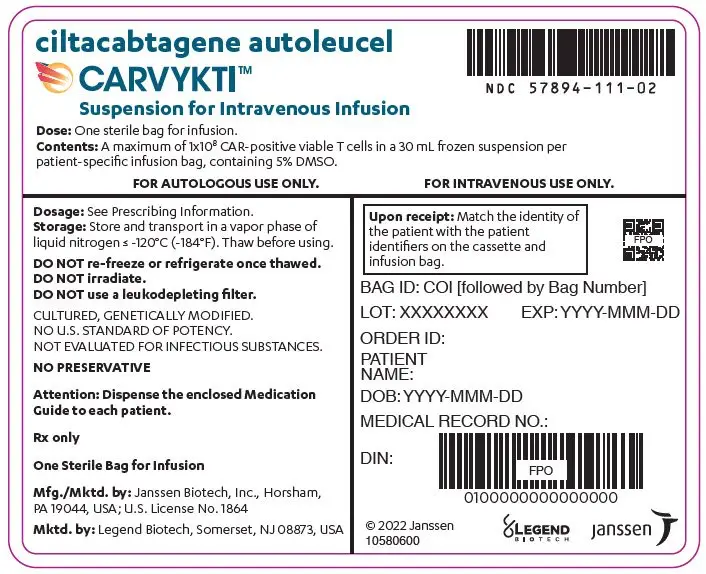
16. How is Carvykti supplied
CARVYKTI is supplied in one infusion bag containing a frozen suspension of genetically modified autologous T cells in 5% DMSO, either as a:
- 70 mL suspension in an infusion bag and metal cassette (NDC 57894-111-01)
or - 30 mL suspension in an infusion bag and metal cassette (NDC 57894-111-02)
Each CARVYKTI infusion bag is individually packed in an aluminum cryo-cassette.
Match the identity of the patient with the patient identifiers on the cassette and infusion bag upon receipt.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure [18%, (17/97 in the clinical study)]. In case of a manufacturing failure, a second manufacturing of CARVYKTI may be attempted. In addition, while the patient awaits the product, additional anticancer treatment (other than lymphodepletion) may be necessary and may increase the risk of adverse reactions during the pre-infusion period, which could delay or prevent the administration of CARVYKTI.
Advise patients that they will be monitored daily for the first 10 days following the infusion at a REMS-certified healthcare facility, and instruct patients to remain within proximity of a certified healthcare facility for at least 4 weeks following the infusion.
Prior to infusion, advise patients of the following risks and to seek immediate medical attention in the event of the following signs or symptoms:
| CARVYKTI
ciltacabtagene autoleucel injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Janssen Biotech, Inc (099091753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceuticals, Inc. | 868441320 | analysis(57894-111) , manufacture(57894-111) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Vaccines, Zweigniederlassung der Cilag GmbH International | 480244564 | manufacture(57894-111) , api manufacture(57894-111) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioReliance Corporation | 147227730 | analysis(57894-111) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Biologics B.V. | 409612918 | analysis(57894-111) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Biotech, Inc. | 038978363 | analysis(57894-111) | |