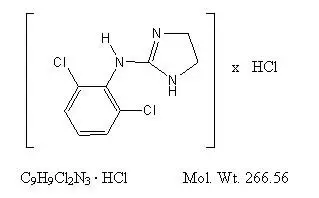
Drug Detail:Catapres (Clonidine (oral) [ kloe-ni-deen ])
Drug Class: Antiadrenergic agents, centrally acting
Related/similar drugs
amlodipine, lisinopril, metoprolol, losartan, furosemide, hydrochlorothiazideIndications and Usage for Catapres
Precautions
Adverse Reactions/Side Effects
Dermatological: Alopecia, angioneurotic edema, hives, pruritus, rash, and urticaria.
Hematologic: Thrombocytopenia.
Musculoskeletal: Leg cramps and muscle or joint pain.
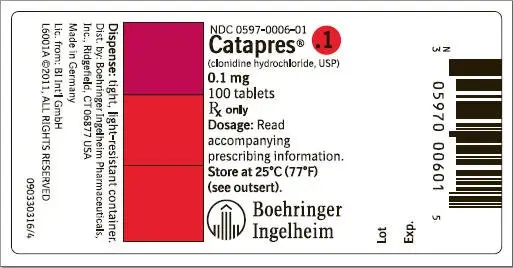
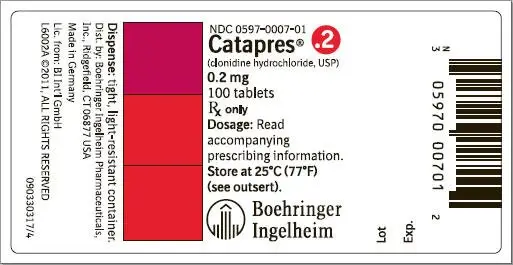
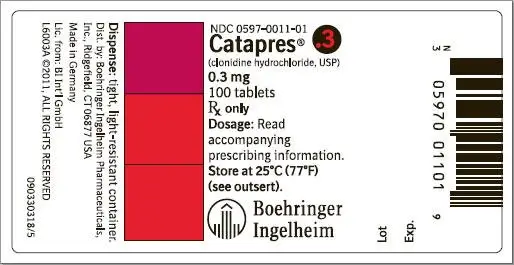
How is Catapres supplied
Catapres® (clonidine hydrochloride, USP) tablets are supplied as follows:
| Dose (mg) | Color | Marking | Bottle of 100 |
| 0.1 | Tan | BI 6 | NDC 0597-0006-01 |
| 0.2 | Orange | BI 7 | NDC 0597-0007-01 |
| 0.3 | Peach | BI 11 | NDC 0597-0011-01 |
| CATAPRES
clonidine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CATAPRES
clonidine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CATAPRES
clonidine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Promeco, S.A. de C.V. | 812579472 | LABEL(0597-0007, 0597-0006, 0597-0011) , PACK(0597-0011, 0597-0007, 0597-0006) , MANUFACTURE(0597-0007, 0597-0006, 0597-0011) , ANALYSIS(0597-0006, 0597-0011, 0597-0007) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | API MANUFACTURE(0597-0007) , ANALYSIS(0597-0007) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West-Ward Columbus Inc. | 058839929 | LABEL(0597-0011) | |