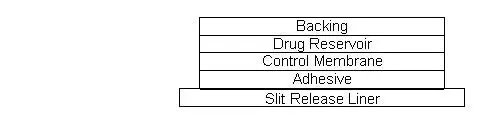
Drug Detail:Catapres-tts (Clonidine (transdermal) [ kloe-ni-deen ])
Drug Class: Antiadrenergic agents, centrally acting
Catapres-TTS Description
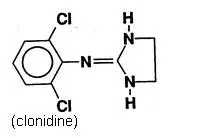
Catapres‑TTS® (clonidine transdermal system) is a transdermal system providing continuous systemic delivery of clonidine for 7 days at an approximately constant rate. Clonidine is a centrally acting alpha‑agonist hypotensive agent. It is an imidazoline derivative with the chemical name 2, 6‑dichloro‑N‑2‑imidazolidinylidenebenzenamine and has the following chemical structure:
Precautions
General
In hypertension caused by pheochromocytoma, no therapeutic effect of Catapres‑TTS® can be expected.
Adverse Reactions/Side Effects
Marketing Experience with Catapres-TTS®
Body as a Whole: Fever; malaise; weakness; pallor; and withdrawal syndrome.
Gastrointestinal: Anorexia and vomiting.
Genitourinary: Difficult micturition; loss of libido; and decreased sexual activity.
Metabolic: Gynecomastia or breast enlargement and weight gain.
Musculoskeletal: Muscle or joint pain; and leg cramps.
Ophthalmological: Blurred vision; burning of the eyes and dryness of the eyes.
Dermatological: Alopecia, angioneurotic edema, hives, pruritus, rash, and urticaria.
Hematologic: Thrombocytopenia.
Musculoskeletal: Leg cramps and muscle or joint pain.
How is Catapres-TTS supplied
| Programmed Delivery Clonidine in vivo | |||||
| Per Day Over 1 Week |
Clonidine |
Size |
Code |
||
| Catapres-TTS®-1 (clonidine transdermal system) NDC 82089-101-34 | 0.1 mg | 2.5 mg | 3.5 cm2 | BI-31 | |
| Catapres-TTS®-2 (clonidine transdermal system) NDC 82089-102-34 | 0.2 mg | 5.0 mg | 7.0 cm2 | BI-32 | |
| Catapres-TTS®-3 (clonidine transdermal system) NDC 82089-103-34 | 0.3 mg | 7.5 mg | 10.5 cm2 | BI-33 |
Storage and Handling
Manufactured by:
Alza Corporation,
Vacaville, CA 95688, USA
Catapres-TTS® is a registered trademark of Boehringer Ingelheim International GmbH used under license
Address medical inquiries to: (866) 380-1080
222068/C
Patient Counseling Information
Catapres-TTS®
(clonidine transdermal system)
The optional white, round ADHESIVE COVER should be applied directly over the PATCH, should the PATCH begin to separate from the skin. The ADHESIVE COVER ensures that the PATCH sticks to the skin. The Catapres‑TTS® PATCH must be replaced with a new one on a fresh skin site if the one in use significantly loosens or falls off.
Each box of Catapres-TTS® contains two types of pouches:

Figure 2
How to Apply the Catapres-TTS® PATCH
- Apply the square, tan Catapres‑TTS® PATCH once a week, preferably at a convenient time on the same day of the week (i.e., prior to bedtime on Tuesday of week one; prior to bedtime on Tuesday of week two, etc.).
- Select a hairless area such as on the upper, outer arm or upper chest. The area chosen should be free of cuts, abrasions, irritation, scars or calluses and should not be shaved before applying the Catapres‑TTS® PATCH. Do not place the Catapres‑TTS® PATCH on skin folds or under tight undergarments, since premature loosening may occur.
- Wash hands with soap and water and thoroughly dry them.
- Clean the area chosen with soap and water. Rinse and wipe dry with a clean, dry tissue.
- Select the pouch with the red and orange colors labeled Catapres-TTS® and open it as illustrated in Figure 3. Remove the square, tan PATCH from the pouch. Figure 3
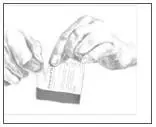
- Remove the clear plastic protective backing from the PATCH by gently peeling off one half of the backing at a time as shown in Figure 4. Avoid touching the sticky side of the Catapres‑TTS® PATCH. Figure 4
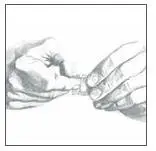
- Place the Catapres‑TTS® PATCH on the prepared skin site (sticky side down) by applying firm pressure over the PATCH to ensure good contact with the skin, especially around the edges (Figure 5). Discard the clear plastic protective backing and wash your hands with soap and water to remove any drug from your hands. Figure 5
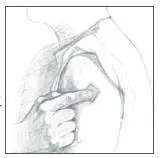
- After one week, remove the old PATCH and discard it (refer to Instructions for Disposal). After choosing a different skin site, repeat instructions 2 through 7 for the application of your next Catapres‑TTS® PATCH.
What to do if your Catapres-TTS® PATCH becomes loose while wearing:
How to Apply the ADHESIVE COVER
- Wash hands with soap and water and thoroughly dry them.
- Using a clean, dry tissue, make sure that the area around the square, tan Catapres-TTS® PATCH is clean and dry. Press gently on the Catapres‑TTS® PATCH to ensure that the edges are in good contact with the skin.
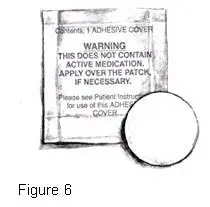
- Take the white, round, ADHESIVE COVER (Figure 6) from the plain white pouch and remove the paper liner backing from the COVER.
- Carefully center the round, white ADHESIVE COVER over the square, tan Catapres‑TTS® PATCH and apply firm pressure, especially around the edges in contact with the skin.
Instructions for Disposal
KEEP OUT OF REACH OF CHILDREN
Manufactured by:
Alza Corporation,
Vacaville, CA 95688 USA
Copyright © 2021 Technomed Inc.
ALL RIGHTS RESERVED
222068/C
PRINCIPAL DISPLAY PANEL - 0.1 mg Adhesive Cover
Contents: 1 ADHESIVE COVER
WARNING
THIS DOES NOT CONTAIN
ACTIVE MEDICATION.
APPLY OVER THE PATCH,
IF NECESSARY.
1

PRINCIPAL DISPLAY PANEL - 0.2 mg Carton
NDC 82089-102-34
Catapres-TTS®-2
(clonidine transdermal system)
4 Systems and 4 Adhesive Covers
Programmed delivery in vivo of 0.2 mg clonidine
per day for one week.
Rx only
For Transdermal Use Only -
See package insert for dosage information

PRINCIPAL DISPLAY PANEL - 0.2 mg Adhesive Cover
Contents: 1 ADHESIVE COVER
WARNING
THIS DOES NOT CONTAIN
ACTIVE MEDICATION.
APPLY OVER THE PATCH,
IF NECESSARY.
2
PRINCIPAL DISPLAY PANEL - 0.2 mg Pouch
Catapres-TTS®-2
(clonidine transdermal system)
Programmed delivery in vivo of 0.2 mg clonidine
per day for one week.
To avoid possible burns, remove the
Catapres-TTS® patch before
undergoing an MRI (magnetic
resonance imaging) procedure.
Rx only
222063


PRINCIPAL DISPLAY PANEL carton - 0.3 mg
NDC 82089-103-34
Catapres-TTS®-3
(clonidine transdermal system)
4 Systems and 4 Adhesive Covers
Programmed delivery in vivo of 0.3 mg clonidine
per day for one week.
Rx only
For Transdermal Use Only -
See package insert for dosage information

| CATAPRES-TTS-1
clonidine transdermal system patch |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| CATAPRES-TTS-2
clonidine transdermal system patch |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| CATAPRES-TTS-3
clonidine transdermal system patch |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Technomed Inc. (116982116) |