Drug Detail:Ceprotin (Protein c concentrate (human))
Drug Class: Miscellaneous coagulation modifiers
Highlights of Prescribing Information
CEPROTIN [Protein C Concentrate (Human)] Lyophilized Powder for Solution for Injection
Initial U.S. Approval: 2007
Indications and Usage for Ceprotin
CEPROTIN, Protein C Concentrate (Human), is an anticoagulant indicated for neonates, pediatric and adult patients with severe congenital Protein C deficiency for the prevention and treatment of venous thrombosis and purpura fulminans. (1)
Ceprotin Dosage and Administration
For intravenous administration only.
- Initiate treatment under the supervision of a physician experienced in using coagulation factors/inhibitors where monitoring of Protein C activity is feasible. (2.1)
- Table provides the CEPROTIN dosing schedule for acute episodes, short-term prophylaxis, and long-term prophylaxis*
Initial Dose* Subsequent 3 Doses* Maintenance Dose* *Dosing is based upon a clinical trial of 15 patients. - *
- Adjust the dose according to the pharmacokinetic profile for each individual patient(2.1).
- †
- Continue CEPROTIN until desired anticoagulation is achieved., NA = Not applicable; Q = every.
Acute Episodes, Short-term Prophylaxis† 100-120 IU/kg 60-80 IU/kg
Q 6 hours45-60 IU/kg
Q 6 or Q 12 hoursLong-term Prophylaxis NA NA 45-60 IU/kg
Q 12 hours - Administer CEPROTIN at a maximum injection rate of 2 mL per minute except for children with a bodyweight of < 10 kg, where the injection rate should not exceed a rate of 0.2 mL/kg/minute.
Dosage Forms and Strengths
 : Approximately 500 IU/vial (3)
: Approximately 500 IU/vial (3)
 : Approximately 1000 IU/vial (3)
: Approximately 1000 IU/vial (3)
Contraindications
None. (4)
Warnings and Precautions
- Discontinue administration of CEPROTIN if symptoms of hypersensitivity/allergic reactions occur. (2.1, 5.1, 6)
- Because CEPROTIN is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease agent. (5.2, 11)
- Simultaneous administration of CEPROTIN with tPA and/or anticoagulants may increase the risk of bleeding. (5.3)
- CEPROTIN contains heparin. If heparin-induced thrombocytopenia is suspected, check platelet counts immediately and discontinue administration. (5.4)
- CEPROTIN contains sodium >200 mg. Inform patients on a low sodium diet and/or patients with renal impairment. (5.5)
Adverse Reactions/Side Effects
Common adverse reactions observed in clinical trials were rash, itching, and lightheadedness. (5.1, 6)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Related/similar drugs
protein cFull Prescribing Information
1. Indications and Usage for Ceprotin
CEPROTIN, Protein C Concentrate (Human), is an anticoagulant indicated for neonates, pediatric and adult patients with severe congenital Protein C deficiency for the prevention and treatment of venous thrombosis and purpura fulminans.
2. Ceprotin Dosage and Administration
For intravenous administration only.
2.1 Dose
- Initiate treatment with CEPROTIN under the supervision of a physician experienced in replacement therapy with coagulation factors/inhibitors where monitoring of protein C activity is feasible.
- The dose, administration frequency, and duration of treatment with CEPROTIN depends on the severity of the protein C deficiency, the patient's age, the clinical condition of the patient, and the patient's plasma level of protein C.
- Adjust the dose regimen according to the pharmacokinetic profile for each individual patient. [See DOSAGE AND ADMINISTRATION: Protein C Activity Monitoring].
Table 1 provides the CEPROTIN dosing schedule for acute episodes, short-term prophylaxis and long-term prophylaxis.
| Initial Dose† | Subsequent 3 Doses† | Maintenance Dose† | |
|---|---|---|---|
| NA = Not applicable; Q = every. | |||
|
|||
| Acute Episode / Short-term Prophylaxis‡ | 100-120 IU/kg | 60 - 80 IU/kg Q 6 hours | 45 - 60 IU/kg Q 6 or 12 hours |
| Long-term Prophylaxis | NA | NA | 45 - 60 IU/kg Q 12 hours |
- Determine protein C recovery and half-life with an initial dose of 100-120 IU/kg in patients receiving treatment for acute episodes and short-term prophylaxis.
- Adjust the dose to maintain a target peak protein C activity of 100 %.
- Continue the patient on the same dose after resolution of the acute episode to maintain trough protein C activity level above 25% for the duration of treatment.
- Patients receiving prophylactic administration of CEPROTIN may warrant higher peak protein C activity levels in situations of an increased risk of thrombosis (such as infection, trauma, or surgical intervention). Therefore it is recommended to maintain trough protein C activity levels above 25%.
- These dosing guidelines are also recommended for neonatal and pediatric patients [See USE IN SPECIFIC POPULATIONS: Pediatric Use (8.4) and CLINICAL PHARMACOLOGY: Pharmacokinetics (12.3)].
Protein C Activity Monitoring
- Determine the patient's protein C plasma level before and during treatment with CEPROTIN by measuring protein C activity using a chromogenic assay.Certain clinical conditions, such as acute thrombosis, purpura fulminans, and skin necrosis, may shorten the half-life of CEPROTIN. See CLINICAL PHARMACOLOGY: Pharmacokinetics (12.3). In case of an acute thrombotic event, immediately measure protein C activity before the next injection until the patient is stable and monitor the protein C levels to maintain the trough protein C level above 25%.
- Patients treated during the acute phase of their disease may display much lower increases in protein C activity. In addition to protein C activity measurement, check the coagulation parameters also;however, data were insufficient to establish correlation between protein C activity levels and coagulation parameters in clinical trials.
Initiation of Vitamin K Antagonists
- In patients starting treatment with oral anticoagulants belonging to the class of vitamin K antagonists, a transient hypercoagulable state may arise before the desired anticoagulant effect becomes apparent. This transient effect may be because protein C, a vitamin K-dependent plasma protein, has a shorter half-life than most of the vitamin K-dependent proteins (i.e., Factor II, IX, and X).
- In the initial phase of treatment, the protein C activity is more rapidly suppressed than that of the procoagulant factors. For this reason, if the patient switched to oral anticoagulants, they must continue protein C replacement until stable anticoagulation is obtained. Although warfarin-induced skin necrosis can occur in any patient during the initiation of treatment with oral anticoagulant therapy, individuals with severe congenital protein C deficiency are particularly at risk.
- During the initiation of oral anticoagulant therapy, it is advisable to start with a low dose of the anticoagulant and adjust this incrementally, rather than use a standard loading dose of the anticoagulant.
3. Dosage Forms and Strengths
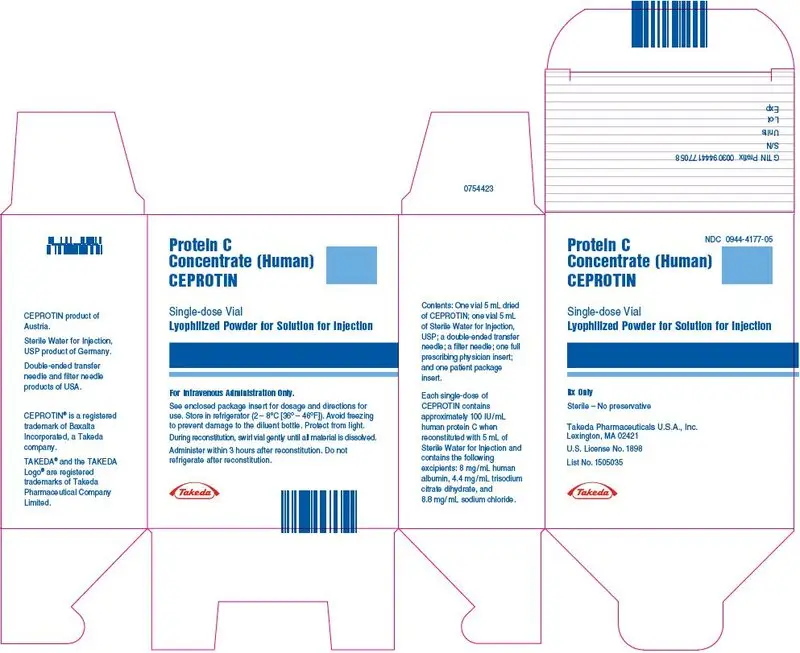
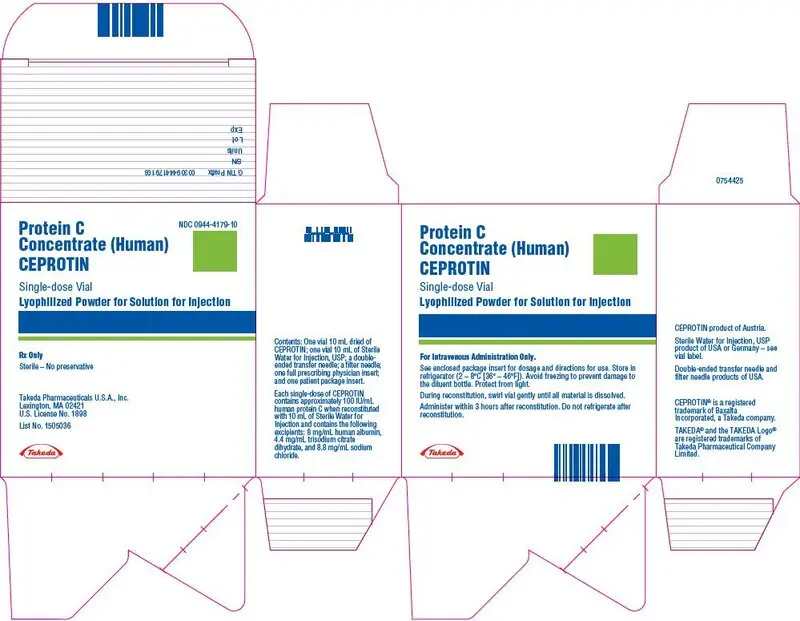
CEPROTIN is available in single-dose vials that contain nominally 500 (blue color bar) or 1000 (green color bar) International Units (IU) human protein C and is reconstituted with 5 mL and 10 mL of Sterile Water for Injection respectively, to provide a single dose of human Protein C at a concentration of 100 IU/mL.
CEPROTIN, when reconstituted with the appropriate volume of diluent, contains the following excipients: 8 mg/mL human albumin, 4.4 mg/mL trisodium citrate dihydrate, and 8.8 mg/mL sodium chloride.
5. Warnings and Precautions
5.1 Hypersensitivity
CEPROTIN may contain traces of mouse protein and/or heparin as a result of the manufacturing process. Allergic reactions to mouse protein and/or heparin cannot be ruled out. If symptoms of hypersensitivity/allergic reaction occur, discontinue the injection/infusion. In case of anaphylactic shock, the current medical standards for treatment are to be observed.
5.2 Transmissible Infectious Agents
Because CEPROTIN is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease agent.
All infections suspected by a physician to have been possibly transmitted by this product should be reported by the physician or other healthcare provider to Takeda Pharmaceuticals at 1-877-TAKEDA-7 (1-877-825-3327). Discuss the risks and benefits of this product with your patient.
5.3 Bleeding Episodes
Several bleeding episodes have been observed in clinical studies. Concurrent anticoagulant medication may have been responsible for these bleeding episodes. However, it cannot be completely ruled out that the administration of CEPROTIN further contributed to these bleeding events.
Simultaneous administration of CEPROTIN and tissue plasminogen activator (tPA) may further increase the risk of bleeding from tPA.
5.4 Heparin-induced Thrombocytopenia (HIT)
CEPROTIN contains trace amounts of heparin which may lead to Heparin-induced Thrombocytopenia, which can be associated with a rapid decrease of the number of thrombocytes. Identifying HIT is complicated because these symptoms may already be present in acute phase patients with severe congenital protein C deficiency. Determine the platelet count immediately and consider discontinuation of CEPROTIN.
6. Adverse Reactions/Side Effects
The common adverse reactions related to CEPROTIN treatment observed were the following hypersensitivity or allergic reactions: lightheadedness and itching and rash.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety profile of CEPROTIN was based on 121 patients from clinical studies and compassionate use in severe congenital Protein C deficiency. Duration of exposure ranged from 1 day to 8 years. One patient experienced hypersensitivity/allergic reactions (itching and rash) and lightheadedness which were determined by the investigator to be related to CEPROTIN.
No inhibiting antibodies to CEPROTIN have been observed in clinical studies. However, the potential for developing antibodies cannot be ruled out.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of CEPROTIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Psychiatric Disorders: Restlessness
Skin and Subcutaneous Tissue Disorders: Hyperhydrosis
General Disorders and Administration Site Conditions: Injection Site Reaction
7. Drug Interactions
No formal drug interaction studies have been conducted.
[See WARNINGS AND PRECAUTIONS: Bleeding Episodes (5.3)] for information regarding simultaneous administration of CEPROTIN and tissue plasminogen activator (tPA).
[See DOSAGE AND ADMINISTRATION: Dose (2.1) Initiation of Vitamin K Antagonists].
8. Use In Specific Populations
8.4 Pediatric Use
Neonatal and pediatric subjects were enrolled during the prospective and retrospective studies described in CLINICAL STUDIES (14). Of the 18 subjects enrolled during the prospective study, 1 was newborn, 3 were between 28 days and 23 months, 9 were between 2 and 11 years, 1 was between 12 and 16 years, and 4 were older than 16 years. Of the 11 subjects enrolled and treated during the retrospective study, 9 were between 2 and 11 years, and 2 were older than 16 years [see CLINICAL STUDIES (14)].
11. Ceprotin Description
CEPROTIN [Protein C Concentrate (Human)] is manufactured from human plasma purified by a combination of filtration and chromatographic procedures, including a column of immobilized mouse monoclonal antibodies on gel beads [See WARNINGS AND PRECAUTIONS: Transmissible Infectious Agents (5.2)].
The manufacturing process for CEPROTIN includes processing steps designed to reduce the risk of viral transmission. The capacity of the manufacturing process to remove and/or inactivate enveloped and non-enveloped viruses has been validated by laboratory spiking studies on a scaled down process model, using the following enveloped and non-enveloped viruses: Human Immunodeficiency Virus Type 1 (HIV-1), Bovine Viral Diarrhea Virus (BVDV ), Tick-Borne Encephalitis Virus (TBEV), Pseudorabies Virus (PRV), Hepatitis A Virus (HAV) and Mice Minute Virus (MMV). Virus reduction steps consist of detergent treatment (Polysorbate 80, P80), heat inactivation (Vapor Heating) and immunoaffinity chromatography (IAX).
Virus clearance studies for CEPROTIN have demonstrated that the process provides for a robust overall virus clearance capacity. A summary of log10 virus reduction factors per virus and manufacturing step is presented in Table 2.
| Manufacturing Step | HIV-1 | HCV Model Viruses | PRV | HAV | MMV | |
|---|---|---|---|---|---|---|
| BVDV | TBEV | |||||
| Abbreviations: IEX, Ion Exchange Chromatography; IAX, Immunoaffinity Chromatography; HIV-1, Human Immunodeficiency Virus Type I; TBEV, Tick-Borne Encephalitis Virus (model for hepatitis C virus [HCV]); BVDV, Bovine Viral Diarrhea Virus (model virus for HCV and other small, enveloped RNA viruses); PRV, Pseudorabies Virus (model virus for enveloped DNA viruses, e.g. HBV, Hepatitis B Virus); HAV, Hepatitis A Virus; MMV, Mice Minute Virus (model for Human Parvovirus B19 and for non enveloped viruses); n.d., not done. | ||||||
|
||||||
| P80 Treatment | >5.1 | >4.7 | n.d. | 2.5* | >3.8* | 1.4* |
| IAX | 3.9 | 2.9 | 3.8 | 4.0 | 0.9 | 3.5 |
| Vapor Heating | 4.6 | >5.9 | n.d. | 5.9 | >4.2 | 1.2 |
12. Ceprotin - Clinical Pharmacology
12.1 Mechanism of Action
Protein C is the precursor of a vitamin K-dependent anticoagulant glycoprotein (serine protease) that is synthesized in the liver [See DOSAGE AND ADMINISTRATION: Dose (2.1) Initiation of Vitamin K Antagonists]. It is converted by the thrombin/thrombomodulin-complex on the endothelial cell surface to activated Protein C (APC). APC is a serine protease with potent anticoagulant effects, especially in the presence of its cofactor protein S. APC exerts its effect by the inactivation of the activated forms of factors V and VIII, which leads to a decrease in thrombin formation. APC has also been shown to have profibrinolytic effects.
The Protein C pathway provides a natural mechanism for control of the coagulation system and prevention of excessive procoagulant responses to activating stimuli. A complete absence of protein C is not compatible with life. A severe deficiency of this anticoagulant protein causes a defect in the control mechanism and leads to unchecked coagulation activation, resulting in thrombin generation and intravascular clot formation with thrombosis.
12.2 Pharmacodynamics
In clinical studies, the intravenous administration of CEPROTIN demonstrated a temporary increase, within approximately half an hour of administration, in plasma levels of APC. Replacement of protein C in protein C-deficient patients is expected to control or, if given prophylactically, to prevent thrombotic complications.
12.3 Pharmacokinetics
Table 3 provides pharmacokinetic results for asymptomatic and symptomatic subjects with protein C deficiency.
| PK parameter | N | Median | 95% CI for median | Min | Max |
|---|---|---|---|---|---|
| Cmax = Maximum concentration after infusion; T max = Time at maximum concentration; AUC 0-Infinity = Area under the curve from 0 to infinity; MRT = Mean residence time; and Incremental recovery = Maximum increase in Protein C concentration following infusion divided by dose | |||||
| Cmax [IU/dL] | 21 | 110 | 106 to 127 | 40 | 141 |
| Tmax [h] | 21 | 0.50 | 0.50 to 1.05 | 0.17 | 1.33 |
| Incremental recovery [(IU/dL)/(IU/kg)] | 21 | 1.42 | 1.32 to 1.59 | 0.50 | 1.76 |
| Initial half-life [h] | 21 | 7.8 | 5.4 to 9.3 | 3.0 | 36.1 |
| Terminal half-life [h] | 21 | 9.9 | 7.0 to 12.4 | 4.4 | 15.8 |
| Half-life by the non-compartmental approach [h] | 21 | 9.8 | 7.1 to 11.6 | 4.9 | 14.7 |
| AUC0-Infinity [IU*h/dL] | 21 | 1500 | 1289 to 1897 | 344 | 2437 |
| MRT [h] | 21 | 14.1 | 10.3 to 16.7 | 7.1 | 21.3 |
| Clearance [dL/kg/h] | 21 | 0.0533 | 0.0428 to 0.0792 | 0.0328 | 0.2324 |
| Volume of distribution at steady state [dL/kg] | 21 | 0.74 | 0.70 to 0.89 | 0.44 | 1.65 |
The protein C plasma activity was measured by chromogenic and/or clotting assay. The maximum plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC) appeared to increase dose-linearly between 40 and 80 IU/kg. The median incremental recovery was 1.42 [(IU/dL)/(IU/kg)] after intravenous administration of CEPROTIN. The median half-lives, based on non-compartmental method, ranged from 4.9 to 14.7 hours, with a median of 9.8 hours. In patients with acute thrombosis, both the increase in protein C plasma levels as well as half-life may be considerably reduced. No formal study or analysis has been performed to evaluate the effect of covariates such as race and gender on the pharmacokinetics of CEPROTIN.
The pharmacokinetic profile in pediatric patients has not been formally assessed. Limited data suggest that the pharmacokinetics of CEPROTIN may be different between very young children and adults. The systemic exposure (Cmax and AUC) may be considerably reduced due to a faster clearance, a larger volume of distribution, and/or a shorter half-life of protein C in very young children than in older subjects. Consider this fact when a dosing regimen for children is determined. Doses should be individualized based upon protein C activity levels [See DOSAGE AND ADMINISTRATION: Dose (2.1) Protein C Activity Monitoring].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Protein C contained in CEPROTIN is a normal constituent of human plasma and acts like endogenous protein C. Studies in heterologous species to evaluate carcinogenicity, reproductive toxicology and developmental toxicology have not been performed.
CEPROTIN has not demonstrated mutagenic potential in the Salmonella Thyphimurium reverse mutation assay (Ames test).
14. Clinical Studies
In a multi-center, open-label, non-randomized study in 3 parts, the safety and efficacy of CEPROTIN was evaluated in subjects with severe congenital protein C deficiency for the (on-demand) treatment of acute thrombotic episodes, such as purpura fulminans (PF), warfarin-induced skin necrosis (WISN) and other thromboembolic events, and for short-term or long-term prophylaxis. Eighteen subjects (9 male and 9 female), ages ranging from 0 (newborn) to 25.7 years participated in this study.
The clinical endpoint of the study was to assess whether episodes of PF and/or other thromboembolic events were treated effectively, effectively with complications, or not treated effectively. Table 4 provides a comparison of the primary efficacy ratings of PF from the study to the historical controls. Inadequate data is available for treatment of WISN.
| Protein C Concentrate (Human) | Historical Controls | ||||
|---|---|---|---|---|---|
| Episode Type | Primary Efficacy Rating | N | % | N | % |
| Purpura Fulminans | Effective | 17 | 94.4 | 11 | 52.4 |
| Effective with Complication | 1 | 5.6 | 7 | 33.3 | |
| Not Effective | 0 | 0.0 | 3 | 14.3 | |
| Total | 18 | 100 | 21 | 100 | |
Of 18 episodes of PF (6 severe, 11 moderate, 1 mild) treated with CEPROTIN for the primary efficacy rating, 17 (94.4%) were rated as effective, and 1 (5.6%) was rated as effective with complications; none (0%) were rated not effective. When compared with the efficacy ratings for 21 episodes of PF (historical control group), subjects with severe congenital protein C deficiency were more effectively treated with CEPROTIN than those treated with modalities such as fresh frozen plasma or conventional anticoagulants.
Table 5 provides a summary of the secondary treatment ratings for treatment of skin lesions and other thrombotic episodes from part one of the study.
| Purpura Fulminans Skin Necrosis | Other Thrombotic Events | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Total | Total | ||||||||
| Rating Category | N | % | N | % | N | % | N | % | N | % | N | % |
| N = Number of episodes | ||||||||||||
| Excellent | 1 | 5.6 | 7 | 38.9 | 5 | 27.8 | 13 | 72.2 | 4 | 80.0 | 17 | 73.9 |
| Good | 0 | 0.0 | 4 | 22.2 | 0 | 0.0 | 4 | 22.2 | 1 | 20.0 | 5 | 21.7 |
| Fair | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 | 1 | 5.6 | 0 | 0 | 1 | 4.3 |
| Total | 1 | 5.6 | 11 | 61.1 | 6 | 33.3 | 18 | 100.0 | 5 | 100.0 | 23 | 100.0 |
In a secondary efficacy rating, 13 (72.2%) of the 18 episodes of PF treated with CEPROTIN were rated as excellent, 4 (22.2%) were rated as good, and 1 (5.6%) episode of severe PF was rated as fair; all were rated as effective. Four (80%) of the 5 episodes of venous thrombosis had treatment ratings of excellent, while 1 (20%) was rated as good.
CEPROTIN was also demonstrated to be effective in reducing the size and number of skin lesions. Non-necrotic skin lesions healed over a maximum 12-day (median 4-day) period and necrotic skin lesions healed over a maximum 52-day (median 11-day) period of CEPROTIN treatment, as shown in Table 6.
| Lesion Type | Number of Episodes (Number of Subjects) | Mean | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Non-necrotic | 16 (9 subjects) | 4.6 | 4.0 | 1 | 12 |
| Necrotic | 7 (5 subjects) | 21.1 | 11.0 | 5 | 52 |
Changes in the extent of venous thrombus were also measured for the 5 thromboembolic episodes. CEPROTIN prevented an increase in the extent of thrombus during 4 (80%) of the thromboembolic episodes by Day 3 of treatment, and 1 (20%) episode by Day 5 of treatment.
All seven of the short-term prophylaxis treatments with CEPROTIN were free of complications of PF or thromboembolic events, as shown in Table 7.
| Reason for Treatment | Number of Treatments | Presentation of Purpura Fulminans During Treatment Episodes | Thromboembolic Complications During Treatment Episode | Number of Treatments Free of Complications | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Anticoagulation Therapy | 3 | 0 | 0.0 | 0 | 0.0 | 3 | 100.0 |
| Surgical Procedure | 4 | 0 | 0.0 | 0 | 0.0 | 4 | 100.0 |
| Total | 7 | 0 | 0.0 | 0 | 0.0 | 7 | 100.0 |
No episodes of PF occurred in four subjects ranging from 42 to 338 days of long-term prophylactic treatment with CEPROTIN, as shown in Table 8. When not on prophylactic treatment and receiving CEPROTIN on-demand, the same four subjects experienced a total of 13 (median of 3) episodes of PF over a range of 19 to 323 days. The time to first episode of PF after exiting from long-term prophylaxis treatment ranged from 12 to 32 days for these four subjects.
| Summary Statistic | Long-Term Prophylactic Treatment | While On-Demand* | Time to First Episode After Existing Long Term Prophylaxis | ||||
|---|---|---|---|---|---|---|---|
| Number of Episodes per Subject | Number of Days Receiving Prophylactic Treatment | Monthly Rate of Episodes | Number of Episodes per Subject | Number of Days Not Receiving Study Drug | Monthly Rate of Episodes | ||
|
|||||||
| Mean | 0 | 229 | 0.0 | 3.3 | 165 | 1.91 | 23.3 |
| Median | 0 | 268 | 0.0 | 3.0 | 159 | 0.49 | 24.5 |
| Minimum | 0 | 42 | 0.0 | 1.0 | 19 | 0.25 | 12.0 |
| Maximum | 0 | 338 | 0.0 | 6.0 | 323 | 6.40 | 32.0 |
16. How is Ceprotin supplied
CEPROTIN, Protein C Concentrate (Human), is supplied as a sterile, white or cream colored, lyophilized powder for IV injection. It has a pH between 6.7 and 7.3 and an osmolality not lower than 240 mosmol/kg. One International Unit (IU) of protein C corresponds to the amidolytically measured activity of protein C in 1 mL of normal plasma. The potency (IU) is determined using a chromogenic substrate method referenced against the World Health Organization (WHO) International Standard (86/622).
CEPROTIN is available in single-dose vials that contain the following nominal product strengths:
 : 500 IU per vial: (NDC: 0944-4177-05)
: 500 IU per vial: (NDC: 0944-4177-05)
 : 1000 IU per vial: (NDC: 0944-4179-10)
: 1000 IU per vial: (NDC: 0944-4179-10)
Actual potency is printed on the vial label.
One package of CEPROTIN contains one glass vial of CEPROTIN powder, one glass vial of Sterile Water for Injection, USP, one transfer needle, one filter needle, one full prescribing physician insert and one patient package insert.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Inform patients of the following:
- Early signs of hypersensitivity reactions include hives, generalized urticaria, tightness of the chest, wheezing, hypotension and anaphylaxis. The risk of an allergic type hypersensitivity reaction cannot be excluded [See WARNING AND PRECAUTIONS: Hypersensitivity (5.1)]. CEPROTIN may contain traces of mouse protein or heparin as a result of the manufacturing process. Allergic reactions to mouse protein or heparin cannot be ruled out. Immediately discontinue the injection/infusion and inform their physician as soon as possible if symptoms of hypersensitivity/allergic reaction occur.
INSTRUCTIONS FOR USE
CEPROTIN [see-PRO-ten]
Protein C Concentrate (Human)
for intravenous use
This "Instructions for Use" contains information on how to inject CEPROTIN.
IMPORTANT: Contact your doctor if you experience any problems with this procedure. These instructions are intended only as an aid for those patients who have been instructed by their doctor on the proper way to self-infuse the product. Do not attempt to self-infuse unless you have been taught how by your doctor.
- Prepare a clean surface and gather all the materials you will need for the infusion. You will need to gather exam gloves (optional), alcohol swabs (or other suitable solution suggested by your doctor), a winged infusion set, and a tourniquet, as these are not provided with your package of CEPROTIN.
- Check the expiration date on the CEPROTIN vial. Do not use CEPROTIN after the expiration date.
- Let the vial of CEPROTIN and the vial of Sterile Water for Injection, USP (diluent) warm up to room temperature.
- Wash your hands and put on clean exam gloves (optional).
- Remove caps from the CEPROTIN and diluent vial to expose the centers of the rubber stoppers.
- Cleanse the stoppers with an alcohol swab (or other suitable solution suggested by your doctor) by rubbing the stoppers firmly for several seconds and allow them to dry.
- Remove the protective covering from one end of the double-ended transfer needle and insert the exposed needle through the center of the diluent vial stopper.
- While keeping the needle in the diluent vial, remove the protective covering from the other end of the double-ended transfer needle.
- Invert the diluent vial over the upright CEPROTIN vial. Then, insert the free end of the needle through the CEPROTIN vial stopper at its center. The vacuum in the vial will draw in the diluent. If there is no vacuum in the CEPROTIN vial, do not use the product. Contact Takeda Pharmaceuticals Customer Service.
- Separate the two vials by removing the needle from the diluent vial stopper. Then, remove the transfer needle from the CEPROTIN vial. Do not attempt to recap the needle and do not dispose it in ordinary household trash. Place the needle in a hard-walled Sharps container for proper disposal.
- Gently swirl the CEPROTIN vial until all the powder is completely dissolved. The solution should be colorless to slightly yellowish and free of visible particles. Do not use the solution if you see particles in it. Administer CEPROTIN at room temperature within 3 hours of mixing.
- Attach the filter needle to a disposable syringe and draw back the plunger to allow air into the syringe. Insert the filter needle into the reconstituted CEPROTIN.
- Inject air into the vial, and then withdraw the solution into the syringe.
- Remove and discard the filter needle from the syringe. Do not attempt to recap the needle and do not dispose it in ordinary household trash. Place the needle in a hard-walled Sharps container for proper disposal.
- Attach a winged infusion set, if available, or a suitable needle (not supplied) for the injection.
- Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe.
- Apply a tourniquet, and prepare the injection site by wiping the skin well with an alcohol swab (or other suitable solution suggested by your doctor).
- Insert the needle into the vein, and remove the tourniquet. Infuse CEPROTIN. CEPROTIN should be administered at a maximum injection rate of 2 milliliters (mL) per minute except for children with a bodyweight of < 10 kg (22 pounds), where the injection rate should not exceed a rate of 0.2 mL per kilogram per minute.
- Remove the needle from the vein and apply pressure with sterile gauze to the infusion site for several minutes. Do not attempt to recap the needle after the infusion, and do not dispose it in ordinary household trash. Place the needle with the used syringe in a hard-walled Sharps container for proper disposal.
- Clean up any blood with a freshly prepared mixture of 1 part bleach and 9 parts water, soap, and water, or any household disinfecting solution.
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898
CEPROTIN® is a registered trademark of Baxalta Incorporated, a Takeda company.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
CEP367
Revised: 3/2023
Important: Contact your doctor if you have any questions or experience any adverse effects. These instructions are intended as an additional aid only for those patients who have been instructed by their doctor on the proper way to self-infuse CEPROTIN. If you have not been instructed to self-infuse by your doctor, do not attempt to self-infuse.
| CEPROTIN
protein c concentrate human kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| CEPROTIN
protein c concentrate human kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda Manufacturing Austria AG | 300434670 | MANUFACTURE(0944-4177, 0944-4179) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Siegfried Hameln GmbH | 315869123 | MANUFACTURE(64764-515, 64764-516) | |




 : Approximate dosage strength of 500 IU per vial.
: Approximate dosage strength of 500 IU per vial. : Approximate dosage strength of 1000 IU per vial.
: Approximate dosage strength of 1000 IU per vial.