Drug Detail:Cerezyme (Imiglucerase [ im-ih-glue-ker-ase ])
Drug Class: Lysosomal enzymes
Highlights of Prescribing Information
CEREZYME ® (imiglucerase) for injection, for intravenous use
Initial U.S. Approval: 1994
Indications and Usage for Cerezyme
Cerezyme is a hydrolytic lysosomal glucocerebrosidase-specific enzyme indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions: anemia, thrombocytopenia, bone disease, hepatomegaly or splenomegaly. (1)
Cerezyme Dosage and Administration
- The recommended dosage ranges from 2.5 units/kg three times a week to 60 units/kg once every two weeks. (2.1)
- Cerezyme is administered by intravenous infusion over 1 to 2 hours. (2.1)
- Titrate the dosage based on clinical manifestations of disease and therapeutic goals for the patient. (2.1)
- See the full prescribing information for preparation and administration instructions. (2.2)
Dosage Forms and Strengths
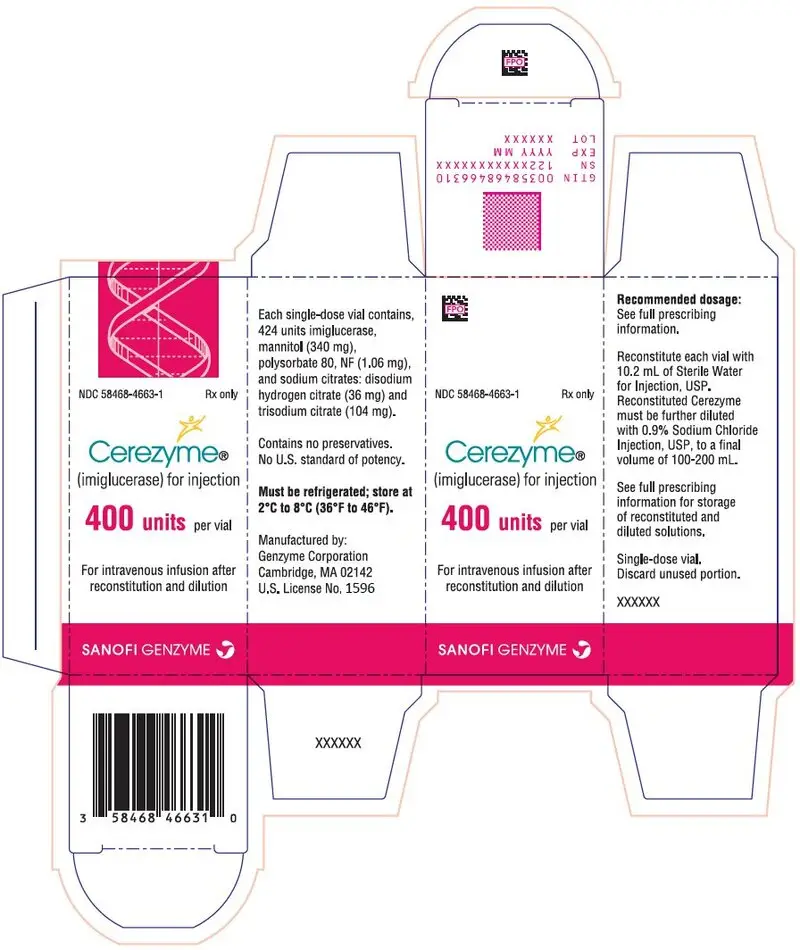
For injection: 400 units of imiglucerase as a lyophilized powder in a single-dose vial. (3)
Contraindications
None. (4)
Warnings and Precautions
- Hypersensitivity and Infusion-Associated Reactions: Consider periodic monitoring of patients during the first year of treatment for IgG antibody formation. If a severe hypersensitivity reaction occurs, discontinue Cerezyme treatment and initiate appropriate medical treatment. (5.1)
Adverse Reactions/Side Effects
- Adverse reactions reported in adults include back pain, chills, dizziness, fatigue, headache, hypersensitivity reactions, nausea, pyrexia, and vomiting. (6.1)
- Adverse reactions reported in pediatric patients 2 years of age and older are similar to adults. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme at 1-800-745-4447 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2022
Related/similar drugs
Doptelet, prednisone, dexamethasone, Decadron, Deltasone, Promacta, CerdelgaFull Prescribing Information
1. Indications and Usage for Cerezyme
Cerezyme is indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions:
- anemia
- thrombocytopenia
- bone disease
- hepatomegaly or splenomegaly
2. Cerezyme Dosage and Administration
2.1 Recommended Dosage
Therapy with Cerezyme should be directed by physicians knowledgeable in the management of patients with Gaucher disease.
The recommended dosage of Cerezyme based upon disease severity ranges from 2.5 units/kg three times a week to 60 units/kg once every two weeks. For patients weighing 18 kg and greater, infuse the diluted Cerezyme solution over 1 to 2 hours. For patients weighing less than 18 kg, infuse the diluted Cerezyme solution over 2 hours [see Dosage and Administration (2.2)]. Titrate the dosage based on clinical manifestations of disease and therapeutic goals for the patient.
For patients who experience hypersensitivity reactions to Cerezyme premedicate with antihistamines and/or corticosteroids. Monitor patients for the occurrence of new hypersensitivity reactions [see Warnings and Precautions (5.1)].
3. Dosage Forms and Strengths
For injection: 400 units of imiglucerase as a white to off-white lyophilized powder in a single-dose vial for reconstitution.
5. Warnings and Precautions
5.1 Hypersensitivity and Infusion-Associated Reactions
Hypersensitivity reactions, some of which are serious and include anaphylaxis, have been reported. In addition, hypersensitivity and other infusion-associated reactions have been reported during or shortly after infusion and include pruritus, flushing, urticaria, angioedema, chest discomfort, dyspnea, cough, cyanosis, tachycardia, and hypotension [see Adverse Reactions (6.1)]. Patients with antibody to imiglucerase have a higher risk of hypersensitivity reactions. Conversely, not all patients with symptoms of hypersensitivity have detectable IgG antibody. Consider periodic monitoring of patients during the first year of treatment for IgG antibody formation [see Adverse Reactions (6.2)].
If a severe hypersensitivity reaction occurs, discontinue Cerezyme treatment and initiate appropriate medical treatment. Consider the risks and benefits of readministering Cerezyme to individual patients following a severe reaction. If the decision is made to readminister the product, consider reducing the rate of infusion and pretreat with antihistamines and/or corticosteroids and monitor patients for the occurrence of new signs and symptoms of a severe hypersensitivity reaction.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials and Postmarketing Experience
The following adverse reactions associated with the use of imiglucerase were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
| System Organ Class | Adverse Reactions |
|---|---|
|
|
| Nervous system disorders | dizziness, headache |
| Cardiac disorders | tachycardia |
| Vascular disorders | cyanosis,* flushing,* hypotension* |
| Respiratory, thoracic and mediastinal disorders | cough,* dyspnea,* pneumonia, pulmonary hypertension |
| Gastrointestinal disorders | abdominal pain, diarrhea, nausea, vomiting |
| Immune system disorders | anaphylaxis,* hypersensitivity |
| Skin and subcutaneous tissue disorders | angioedema,* pruritus,* rash, urticaria* |
| Musculoskeletal and connective tissue disorders | back pain |
| General disorders and administration site conditions | chest discomfort,* chills, fatigue, infusion-site burning, infusion-site discomfort, infusion-site swelling, pyrexia |
Adverse reactions reported in pediatric patients 2 years of age and older were similar to adults.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other imiglucerase products may be misleading.
Approximately 15% of patients treated and tested to date have developed IgG antibody to Cerezyme during the first year of therapy. Patients who developed IgG antibody did so largely within 6 months of treatment and rarely developed antibodies to Cerezyme after 12 months of therapy. Approximately 46% of patients with detectable IgG antibodies experienced symptoms of hypersensitivity. Patients with antibody to Cerezyme have higher risk of hypersensitivity reaction [see Warnings and Precautions (5.1)]. Patients who developed IgG antibody to Cerezyme had increased elimination half-life compared to patients without antibody [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Cerezyme for treatment of Type 1 Gaucher disease that results in one or more of the following conditions: anemia, thrombocytopenia, bone disease, hepatomegaly or splenomegaly have been established in pediatric patients 2 years of age and older. Use of Cerezyme for this indication is supported by evidence from adequate and well-controlled studies of Cerezyme and alglucerase in adults and pediatric patients 12 years of age and older, with additional data obtained from the medical literature and from postmarketing experience in pediatric patients as young as 2 years of age [see Adverse Reactions (6.1), Clinical Studies (14)].
The safety and effectiveness of Cerezyme have not been established in pediatric patients younger than 2 years of age.
11. Cerezyme Description
Imiglucerase is a hydrolytic lysosomal glucocerebrosidase-specific enzyme. It is an analogue of the human enzyme b-glucocerebrosidase (b-D-glucosyl-N-acylsphingosine glucohydrolase, E.C. 3.2.1.45), produced by recombinant DNA technology using mammalian cell culture (Chinese hamster ovary). Purified imiglucerase is a monomeric glycoprotein of 497 amino acids, containing 4 N-linked glycosylation sites (Mr=60,430). Imiglucerase differs from placental glucocerebrosidase by one amino acid at position 495, where histidine is substituted for arginine. The oligosaccharide chains at the glycosylation sites have been modified to terminate in mannose sugars. The modified carbohydrate structures on imiglucerase are somewhat different from those on placental glucocerebrosidase.
Cerezyme (imiglucerase) for injection is intended for intravenous use. It is supplied as a sterile, nonpyrogenic, white to off-white lyophilized powder for reconstitution with Sterile Water for Injection, USP. Each single-dose vial contains 424 units imiglucerase, mannitol (340 mg), polysorbate 80, NF (1.06 mg), and sodium citrates: disodium hydrogen citrate (36 mg) and trisodium citrate (104 mg).
An enzyme unit (U) is defined as the amount of enzyme that catalyzes the hydrolysis of 1 micromole of the synthetic substrate para-nitrophenyl-b-D-glucopyranoside (pNP-Glc) per minute at 37°C. Reconstituted solutions have a pH of approximately 6.1.
12. Cerezyme - Clinical Pharmacology
12.1 Mechanism of Action
Gaucher disease is characterized by a deficiency of β-glucocerebrosidase activity, which results in accumulation of glucocerebroside in various tissues including liver, spleen, and bone marrow. The mannose sugars on imiglucerase mediate binding to and internalization by cells including macrophages. Cerezyme catalyzes the hydrolysis of glucocerebroside to glucose and ceramide.
12.3 Pharmacokinetics
During one-hour intravenous infusions of four doses (7.5, 15, 30, 60 units/kg) of Cerezyme, steady-state enzymatic activity was achieved by 30 minutes. Following infusion, the half-life of plasma enzymatic activity ranged from 3.6 to 10.4 minutes. Plasma clearance ranged from 9.8 to 20.3 mL/min/kg (mean ± SD, 14.5 ± 4.0 mL/min/kg). The volume of distribution corrected for weight ranged from 0.09 to 0.15 L/kg (mean ± SD, 0.12 ± 0.02 L/kg). These variables do not appear to be influenced by dose or duration of infusion. However, only one or two patients were studied at each dose level and infusion rate.
14. Clinical Studies
Study RC 91-0110 was a randomized, double-blind, active-controlled study of 30 patients (17 male and 13 female), aged 12 to 69 years (mean age of 38 years in the Cerezyme group and mean age of 28 years in the alglucerase group at baseline), with Gaucher disease type 1 and a hemoglobin of at least 1 g/dL below the lower age limit for age and sex. Patients were randomized 1:1 to receive either Cerezyme 60 units/kg every other week or alglucerase for 6 months. Primary efficacy parameters were an increase in hemoglobin concentration of at least 1 g/dL, increase in platelet count and decrease in spleen and liver volume at 6 months. Efficacy results are shown in Table 1.
| Clinical Parameter | Cerezyme (N=15) | Alglucerase (N=15) | Difference (Cerezyme – Alglucerase) [95% CI]* |
|
|---|---|---|---|---|
|
||||
| Hemoglobin concentration (g/dL) | Baseline | 10.7 | 10.9 | – |
| Absolute Change from Baseline | 1.9 | 1.6 | 0.3 [-0.6, 1.3] | |
| Platelet count (× 103/mL3) | Baseline | 68.5 | 74.2 | – |
| Absolute Change from Baseline | 22.7 | 15.8 | 6.9 [-10.4, 24.1] | |
| Liver volume (mL) | Baseline | 2521 | 2788 | – |
| Absolute Change from Baseline | -310 | -307 | -3 [-246, 240] | |
| Percent Change from Baseline (%) | -11 | -10 | -1 [-9, 7] | |
| Spleen volume (mL) | Baseline | 2369 | 2603 | – |
| Absolute Change from Baseline | -902 | -874 | -28 [-652, 596] | |
| Percent Change from Baseline (%) | -35 | -30 | -5 [-14, 4] | |
Bone x-rays showed improvements in cortical thickness and lucencies in 7 of 11 Cerezyme treated patients.
In study RC 92-0501, twenty-nine patients continued treatment for total duration of 24 months. Patients were unblinded at 9 months and allowed to cross-over to Cerezyme treatment. At 24 months, mean increase in hemoglobin was 2.4 g/dL, mean increase in platelet count was 40 ×103/mL3, mean change in liver volume was -20%, and mean change in spleen volume was -57%.
16. How is Cerezyme supplied
Cerezyme (imiglucerase) for injection, 400 units as a white to off-white lyophilized powder in a single-dose vial: NDC 58468-4663-1
| CEREZYME
imiglucerase injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genzyme Corporation (025322157) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Resilience US, Inc | 118999964 | ANALYSIS(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Ireland Limited | 985127419 | ANALYSIS(58468-4663) , MANUFACTURE(58468-4663) , PACK(58468-4663) , LABEL(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 050424395 | PACK(58468-4663) , LABEL(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Charles River Laboratories, Inc. | 078495006 | ANALYSIS(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Biopharma Product Testing Ireland Limited | 238239933 | ANALYSIS(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 117450412 | ANALYSIS(58468-4663) , MANUFACTURE(58468-4663) , API MANUFACTURE(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 968302658 | ANALYSIS(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 034378252 | ANALYSIS(58468-4663) , MANUFACTURE(58468-4663) , API MANUFACTURE(58468-4663) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lonza Biologics, Inc. | 093149750 | ANALYSIS(58468-4663) , API MANUFACTURE(58468-4663) | |