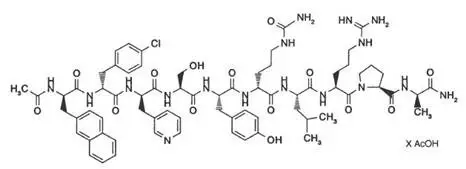
Drug Detail:Cetrotide (injectable) (Cetrorelix (injectable) [ se-troe-rel-ix ])
Drug Class: Gonadotropin-releasing hormone antagonists
Related/similar drugs
Clomid, clomiphene, HCG, Pregnyl, chorionic gonadotropin (hcg), OvidrelCetrotide - Clinical Pharmacology
GnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2) feedback at midcycle, GnRH liberation is enhanced resulting in an LH-surge. This LH-surge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and subsequently luteinization as indicated by rising progesterone levels.
Cetrotide® competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner. The onset of LH suppression is approximately one hour with the 3 mg dose and two hours with the 0.25 mg dose. This suppression is maintained by continuous treatment and there is a more pronounced effect on LH than on FSH. An initial release of endogenous gonadotropins has not been detected with Cetrotide®, which is consistent with an antagonist effect.
The effects of Cetrotide® on LH and FSH are reversible after discontinuation of treatment. In women, Cetrotide® delays the LH-surge, and consequently ovulation, in a dose-dependent fashion. FSH levels are not affected at the doses used during controlled ovarian stimulation. Following a single 3 mg dose of Cetrotide®, duration of action of at least 4 days has been established. A dose of Cetrotide® 0.25 mg every 24 hours has been shown to maintain the effect.
Pharmacokinetics
The pharmacokinetic parameters of single and multiple doses of Cetrotide® (cetrorelix acetate for injection) in adult healthy female subjects are summarized in Table 1.
| Single dose 3 mg | Single dose 0.25 mg | Multiple dose 0.25 mg | |
|---|---|---|---|
| tmax Time to reach observed maximum plasma concentration t1/2 Elimination half-life Cmax Maximum plasma concentration; multiple dose Css, max AUC Area under the curve; single dose AUC0-inf, multiple dose AUCt CL Total plasma clearance Vz Volume of distribution Geometric mean (95% CIln), |
|||
|
|||
| No. of subjects | 12 | 12 | 12 |
| tmax* [h] | 1.5 (0.5-2) | 1.0 (0.5-1.5) | 1.0 (0.5-2) |
| t1/2* [h] | 62.8 (38.2-108) | 5.0 (2.4-48.8) | 20.6 (4.1-179.3) |
| Cmax [ng/ml] | 28.5 (22.5-36.2) | 4.97 (4.17-5.92) | 6.42 (5.18-7.96) |
| AUC [ng∙h/ml] | 536 (451-636) | 31.4 (23.4-42.0) | 44.5 (36.7-54.2) |
| CL† [ml/min∙kg] | 1.28‡ | ||
| Vz† [l/kg] | 1.16‡ | ||
Clinical Studies
Seven hundred thirty two (732) patients were treated with Cetrotide® (cetrorelix acetate for injection) in five (two Phase 2 dose-finding and three Phase 3) clinical trials. The clinical trial population consisted of Caucasians (95.5%) and Black, Asian, Arabian and others (4.5%). Women were between 19 and 40 years of age (mean: 32). The studies excluded subjects with polycystic ovary syndrome (PCOS), subjects with low or no ovarian reserve, and subjects with stage III-IV endometriosis.
Two dose regimens were investigated in these clinical trials, either a single dose per treatment cycle or multiple dosing. In the Phase 2 studies, a single dose of 3 mg was established as the minimal effective dose for the inhibition of premature LH surges with a protection period of at least 4 days. When Cetrotide® is administered in a multidose regimen, 0.25 mg was established as the minimal effective dose. The extent and duration of LH-suppression is dose dependent.
In the Phase 3 program, efficacy of the single 3 mg dose regimen of Cetrotide® and the multiple 0.25 mg dose regimen of Cetrotide® was established separately in two adequate and well controlled clinical studies utilizing active comparators. A third non-comparative clinical study evaluated only the multiple 0.25 mg dose regimen of Cetrotide®. The ovarian stimulation treatment with recombinant FSH or human menopausal gonadotropin (hMG) was initiated on day 2 or 3 of a normal menstrual cycle. The dose of gonadotropins was administered according to the individual patient's disposition and response.
In the single dose regimen study, Cetrotide® 3 mg was administered on the day of controlled ovarian stimulation when adequate estradiol levels (400 pg/mL) were obtained, usually on day 7 (range day 5-12). If hCG was not given within 4 days of the 3 mg dose of Cetrotide®, then 0.25 mg of Cetrotide® was administered daily beginning 96 hours after the 3 mg injection until and including the day of hCG administration.
In the two multiple dose regimen studies, Cetrotide® 0.25 mg was started on day 5 or 6 of COS. Both gonadotropins and Cetrotide® were continued daily (multiple dose regimen) until the injection of human chorionic gonadotropin (hCG).
Oocyte pick-up (OPU) followed by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) as well as embryo transfer (ET) were subsequently performed. The results for Cetrotide® are summarized below in Table 2.
| Parameter | Cetrotide® 3 mg (sd, active comparator study) | Cetrotide® 0.25 mg (md, active comparator study) | Cetrotide® 0.25 mg (md, non-comparative study) |
|---|---|---|---|
|
|||
| No. of subjects | 115 | 159 | 303 |
| hCG administered [%] | 98.3 | 96.2 | 96.0 |
| Oocyte pick-up [%] | 98.3 | 94.3 | 93.1 |
| LH-surge [%] (LH ≥ 10 U/L and P* ≥ 1 ng/mL) † | 0.0 | 1.9 | 1.0 |
| Serum E2 [pg/ml] at day hCG‡, § | 1125 (470-2952) | 1064 (341-2531) | 1185 (311-3676) |
| Serum LH [U/L] at day hCG‡, § | 1.0 (0.5-2.5) | 1.5 (0.5-7.6) | 1.1 (0.5-3.5) |
| No. of follicles ≥ 11 mm at day hCG¶ | 11.2±5.5 | 10.8±5.2 | 10.4±4.5 |
| No. of oocytes: IVF¶
ICSI¶ | 9.2±5.2 10.0±4.2 | 7.6±4.3 10.1±5.6 | 8.5±5.1 9.3±5.9 |
| Fertilization rate: IVF¶
ICSI¶ | 0.48±0.33 0.66±0.29 | 0.62±0.26 0.63±0.29 | 0.60±0.26 0.61±0.25 |
| No. of embryos transferred¶ | 2.6±0.9 | 2.1±0.6 | 2.7±1.0 |
| Clinical pregnancy rate [%] | |||
| per attempt | 22.6 | 20.8 | 19.8 |
| per subject with ET | 26.3 | 24.1 | 23.3 |
In addition to IVF and ICSI, one pregnancy was obtained after intrauterine insemination. In the five Phase 2 and Phase 3 clinical trials, 184 pregnancies have been reported out of a total of 732 patients (including 21 pregnancies following the replacement of frozen-thawed embryos).
In the 3 mg regimen, 9 patients received an additional dose of 0.25 mg of Cetrotide® and two other patients received two additional doses of 0.25 mg Cetrotide®. The median number of days of Cetrotide® multiple dose treatment was 5 (range 1-15) in both studies.
No drug related allergic reactions were reported from these clinical studies.
Contraindications
Cetrotide® (cetrorelix acetate for injection) is contraindicated under the following conditions:
- Hypersensitivity to cetrorelix acetate, extrinsic peptide hormones or mannitol.
- Known hypersensitivity to GnRH or any other GnRH analogs.
- Known or suspected pregnancy, and lactation (see PRECAUTIONS).
- Severe renal impairment
Adverse Reactions/Side Effects
The safety of Cetrotide® (cetrorelix acetate for injection) in 949 patients undergoing controlled ovarian stimulation in clinical studies was evaluated. Women were between 19 and 40 years of age (mean: 32). 94.0% of them were Caucasian. Cetrotide® was given in doses ranging from 0.1 mg to 5 mg as either a single or multiple dose.
Table 3 shows systemic adverse events, reported in clinical studies without regard to causality, from the beginning of Cetrotide® treatment until confirmation of pregnancy by ultrasound at an incidence ≥ 1% in Cetrotide® treated subjects undergoing COS.
| (WHO preferred term) | Cetrotide® N=949 % (n) |
|---|---|
|
|
| Ovarian Hyperstimulation Syndrome* | 3.5 (33) |
| Nausea | 1.3 (12) |
| Headache | 1.1 (10) |
Local site reactions (e.g. redness, erythema, bruising, itching, swelling, and pruritus) were reported. Usually, they were of a transient nature, mild intensity and short duration. During post-marketing surveillance, cases of mild to moderate Ovarian Hyperstimulation syndrome and infrequent cases of hypersensitivity reactions including anaphylactoid reactions have been reported.
Two stillbirths were reported in Phase 3 studies of Cetrotide®.
| CETROTIDE
cetrorelix acetate kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - EMD Serono, Inc. (088514898) |