Drug Detail:Cholbam (Cholic acid [ koe-lik-as-id ])
Drug Class: Digestive enzymes
Highlights of Prescribing Information
CHOLBAM (cholic acid) capsules, for oral use
Initial U.S. Approval: 2015
Indications and Usage for Cholbam
CHOLBAM is a bile acid indicated for:
- Treatment of bile acid synthesis disorders due to single enzyme defects (SEDs). (1.1)
- Adjunctive treatment of peroxisomal disorders (PDs) including Zellweger spectrum disorders in patients who exhibit manifestations of liver disease, steatorrhea or complications from decreased fat-soluble vitamin absorption. (1.2)
Limitations of use:
The safety and effectiveness of CHOLBAM on extrahepatic manifestations of bile acid synthesis disorders due to SEDs or PDs including Zellweger spectrum disorders have not been established. (1.3).
Cholbam Dosage and Administration
- The recommended dosage is 10 to 15 mg/kg once daily or in two divided doses, in pediatric patients and adults. See prescribing information for weight-based dosing tables. (2.1)
- The recommended dosage in patients with concomitant familial hypertriglyceridemia is 11 to 17 mg/kg once daily or in two divided doses and is adjusted based on clinical response (2.1)
- Monitor AST, ALT, GGT, alkaline phosphatase, bilirubin and INR every month for the first 3 months, every 3 months for the next 9 months, every 6 months during the next three years and annually thereafter. Administer the lowest dose that effectively maintains liver function. (2.2)
- Discontinue CHOLBAM if liver function does not improve within 3 months of starting treatment, if complete biliary obstruction develops, or if there are persistent clinical or laboratory indicators of worsening liver function or cholestasis; continue to monitor liver function and consider restarting at a lower dose when parameters return to baseline. (2.2, 5.1)
Administration Instructions:
- Take with food. (2.3)
- Do not crush or chew the capsules. For patients unable to swallow the capsules, the capsules can be opened and the contents mixed with drink/food (2.3)
Dosage Forms and Strengths
Capsules: 50 mg, 250 mg (3)
Contraindications
None (4)
Warnings and Precautions
Exacerbation of Liver Impairment: Monitor liver function. Discontinue CHOLBAM if liver function worsens while on treatment. (5.1)
Adverse Reactions/Side Effects
Most common adverse reactions (≥1%) are diarrhea, reflux esophagitis, malaise, jaundice, skin lesion, nausea, abdominal pain, intestinal polyp, urinary tract infection, and peripheral neuropathy. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Travere Therapeutics, Inc. at 1-877-659-5518 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Bile Salt Efflux Pump (BSEP) Inhibitors (e.g., cyclosporine): Avoid concomitant use; if concomitant use is necessary, monitor serum transaminases and bilirubin (7.1)
- Bile Acid Resins and Aluminum-Based Antacids: Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin or aluminum-based antacids. (2.3, 7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
Related/similar drugs
cholic acidFull Prescribing Information
1. Indications and Usage for Cholbam
2. Cholbam Dosage and Administration
2.1 Dosage Regimen for Bile Acid Synthesis Disorders Due to SEDs and PDs Including Zellweger Spectrum Disorders
| 10 mg/kg/day Dosage | ||
|---|---|---|
| Body Weight (kg) | Number of 50 mg capsules | Number of 250 mg capsules |
| 4 to 6 | 1 | 0 |
| 7 to 10 | 2 | 0 |
| 11 to 15 | 3 | 0 |
| 16 to 20 | 4 | 0 |
| 21 to 25 | 0 | 1 |
| 26 to 30 | 1 | 1 |
| 31 to 35 | 2 | 1 |
| 36 to 40 | 3 | 1 |
| 41 to 45 | 4 | 1 |
| 46 to 50 | 0 | 2 |
| 51 to 55 | 1 | 2 |
| 56 to 60 | 2 | 2 |
| 61 to 65 | 3 | 2 |
| 66 to 70 | 4 | 2 |
| 71 to 75 | 0 | 3 |
| 76 to 80 | 1 | 3 |
| 15 mg/kg/day Dosage | ||
|---|---|---|
| Body Weight (kg) | Number of 50 mg capsules | Number of 250 mg capsules |
| 4 to 5 | 1 | 0 |
| 6 to 9 | 2 | 0 |
| 10 to 13 | 3 | 0 |
| 14 to 16 | 4 | 0 |
| 17 to 19 | 0 | 1 |
| 20 to 23 | 1 | 1 |
| 24 to 26 | 2 | 1 |
| 27 to 29 | 3 | 1 |
| 30 to 33 | 4 | 1 |
| 34 to 36 | 0 | 2 |
| 37 to 39 | 1 | 2 |
| 40 to 43 | 2 | 2 |
| 44 to 46 | 3 | 2 |
| 47 to 49 | 4 | 2 |
| 50 to 53 | 0 | 3 |
| 54 to 56 | 1 | 3 |
| 57 to 59 | 2 | 3 |
| 60 to 63 | 3 | 3 |
| 64 to 66 | 4 | 3 |
| 67 to 69 | 0 | 4 |
| 70 to 73 | 1 | 4 |
| 74 to 76 | 2 | 4 |
| 77 to 79 | 3 | 4 |
| 80 | 4 | 4 |
2.3 Administration Instructions
- Take CHOLBAM with food.
- Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin or aluminum-based antacid.
- Do not crush or chew the capsules.
- For patients unable to swallow the capsules, open the capsules and mix the contents with infant formula or expressed breast milk (for younger children), or soft food such as mashed potatoes or apple puree (for older children and adults) in order to mask any unpleasant taste:
- Hold the capsule over the prepared liquid/food, gently twist open, and allow the contents to fall into the liquid/food.
- Mix the entire capsule contents with one or two tablespoons (15 mL to 30 mL) of infant formula, expressed breast milk, or soft food such as mashed potatoes or apple puree.
- Stir for 30 seconds.
- The capsule contents will remain as fine granules in the milk or food and will not dissolve.
- Administer the mixture immediately
3. Dosage Forms and Strengths
CHOLBAM is available in two capsule strengths:
- 50 mg capsule: Size number 2 Swedish orange capsule with cap imprinted with "50mg" and body imprinted with "ASK001". The capsules contain a white to off-white powder.
- 250 mg capsule: Size number 0 white capsule with a cap imprinted with "250mg" and body imprinted with "ASK002". The capsules contain a white to off-white powder.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reaction is described elsewhere in the labeling:
- Exacerbation of Liver Impairment [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Clinical safety experience with CHOLBAM consists of:
- Trial 1: a non-randomized, open-label, single-arm trial of 50 patients with bile acid synthesis disorders due to SEDs and 29 patients with PDs including Zellweger spectrum disorders. Safety data are available over the 18 years of the trial.
- Trial 2: an extension trial of 12 new patients (10 SED and 2 PD) along with 31 (21 SED and 10 PD) patients who rolled over from Trial 1. Safety data are available for 3 years and 11 months of treatment.
| Adverse Reactions | Trial 1 | Trial 2 * | Overall n (%) |
|---|---|---|---|
|
|||
| Diarrhea | 1 | 2 * | 3 (2) |
| Reflux Esophagitis | 1 | 0 | 1 (1) |
| Malaise | 1 | 0 | 1 (1) |
| Jaundice | 1 | 0 | 1 (1) |
| Skin lesion | 1 | 0 | 1 (1) |
| Nausea | 0 | 1 * | 1 (1) |
| Abdominal Pain | 0 | 1 * | 1 (1) |
| Intestinal Polyp | 0 | 1 * | 1 (1) |
| Urinary Tract Infection | 0 | 1 * | 1 (1) |
| Peripheral Neuropathy | 0 | 1 | 1 (1) |
6.2 Postmarketing Experience
- Gastrointestinal disorders: discomfort and distention, emesis, constipation
- General disorders and administrative site conditions: pyrexia/fever
- Skin and subcutaneous tissue disorders: rash
8. Use In Specific Populations
8.1 Pregnancy
11. Cholbam Description
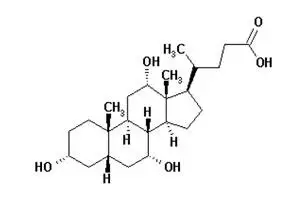
The size 2 capsule shells contain gelatin, red iron oxide and titanium dioxide, and the size 0 capsule shells contain gelatin and titanium dioxide. CHOLBAM is administered orally.
12. Cholbam - Clinical Pharmacology
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Bile Acid Synthesis Disorders due to Single Enzyme Defects
- Trial 1: a non-randomized, open-label, single-arm trial in 50 patients over an 18-year period.
- Trial 2: an extension trial of 12 new patients along with 21 patients who rolled-over from Trial 1 (n=33 total). Efficacy data are available for 21 months of treatment.
- A published case series of 15 patients patients with SEDs and 3 patients with PDs.
Response to CHOLBAM treatment was assessed by the following laboratory criteria:
- ALT or AST values reduced to less than 50 U/L, or baseline levels reduced by 80%;
- total bilirubin values reduced to less than or equal to 1 mg/dL; and
- no evidence of cholestasis on liver biopsy;
and the following clinical criteria:
- body weight increased by 10% or stable at greater than the 50th percentile; and
- survival for greater than 3 years on treatment or alive at the end of Trial 2
CHOLBAM responders were defined as patients who either:
- met at least two laboratory criteria and were alive at the last follow-up; or
- met at least one laboratory criterion, had increased body weight and were alive at the last follow-up.
Overall, 28 of 44 patients (64%) were responders. The breakdown by defect type is as follows:
| Single Enzyme Defect | Responders/Number Treated (%) |
|---|---|
|
|
| 3β-HSD | 22/37 (59%) |
| AKR1D1 | 3/4 (75%) |
| CTX | 2/2 (100%) |
| AMACR | 1/1 (100%) |
| CYP7A1 | N/A* |
| Smith-Lemli-Opitz | N/A* |
14.2 Peroxisomal Disorders including Zellweger Spectrum Disorders
- Trial 1 treated 29 patients with PDs over an 18‑year period.
- Trial 2 treated 2 new patients along with 10 patients who rolled over from Trial 1 (n=12 total). Efficacy data are available from Trial 2 for 21 months of treatment.
- Additional efficacy data were obtained from published case reports of 3 patients.
Response to CHOLBAM treatment was assessed by the following laboratory criteria:
- ALT or AST values reduced to less than 50 U/L, or baseline levels reduced by 80%;
- total bilirubin values reduced to less than or equal to 1 mg/dL; and
- no evidence of cholestasis on liver biopsy;
and the following clinical criteria:
- body weight increased by 10% or stable at greater than the 50th percentile; and
- survival for greater than 3 years on treatment or alive at the end of Trial 2
CHOLBAM responders were defined as patients who either:
- met at least two laboratory criteria and were alive at the last follow-up; or
- met at least one laboratory criterion, had increased body weight and were alive at the last follow-up.
Overall, 11 of 24 patients (46%) were responders. The breakdown by disorder is as follows:
| Peroxisomal Disorder | Responders/Number Treated (%) |
|---|---|
| Neonatal Adrenoleukodystropyhy | 3/6 (50%) |
| Generalized Peroxisomal Disorder | 1/1 (100%) |
| Refsum Disease | 3/4 (75%) |
| Zellweger Syndrome | 3/8 (38%) |
| Peroxisomal Disorder, Type Unknown | 1/5 (20%) |
17. Patient Counseling Information
Exacerbation of Liver Impairment [see Warnings and Precautions (5.1)]
- Advise patients that they will need to undergo laboratory testing periodically while on treatment to assess liver function.
- Advise patients that CHOLBAM may worsen liver impairment and that they should immediately report to their health care provider any symptoms associated with liver impairment (e.g., yellowing of the skin or the whites of the eye, dark or tea-colored urine, pain on the right side of stomach, bleeding or bruising occurs more easily than normal, or increased lethargy)
Administration [see Dosage and Administration (2.3)]
- to take CHOLBAM with food.
- to take CHOLBAM at least one hour before or 4 to 6 hours after taking a bile acid binding resin or an aluminum-based antacid.
- not to crush or chew the capsules.
- for infants and children who cannot swallow capsules, the capsules can be opened and the contents mixed with either infant formula or expressed breast milk (for younger children), or soft food such as mashed potatoes or apple puree (for older children and adults) in order to mask any unpleasant taste:
- Hold the capsule over the prepared liquid/food, gently twist open, and allow the contents to fall into the liquid/food.
- Mix the entire capsule contents with one or two tablespoonfuls (15 mL to 30 mL) of infant formula, expressed breast milk, or soft food such as mashed potatoes or apple puree.
- Stir for 30 seconds.
- The capsule contents will remain as fine granules in the milk or food and will not dissolve.
- Administer the mixture immediately.
CHOLBAM® is a registered trademark of Travere Therapeutics, Inc.
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle Label
NDC 45043- 001-02
90 capsules
Cholbam™
(cholic acid) capsules
50 mg
Rx Only
Manufactured for:
Manchester Pharmaceuticals, Inc.,
San Diego, CA 92130
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
For product information please call 844-246-5226.
PRINCIPAL DISPLAY PANEL - 250 mg Capsule Bottle Label
NDC 45043- 002-02
90 capsules
Cholbam™
(cholic acid) capsules
250 mg
Rx Only
Manufactured for:
Manchester Pharmaceuticals, Inc.,
San Diego, CA 92130
Manufactured by:
Patheon France SA
38300 Bourgoin-Jallieu, France
For product information please call 844-246-5226.

| CHOLBAM
cholic acid capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| CHOLBAM
cholic acid capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Manchester Pharmaceuticals, LLC (832417641) |
| Registrant - Travere Therapeutics, Inc (965454502) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon France S.A.S | 543127229 | manufacture(45043-002, 45043-001) | |




