Drug Class: Otic steroids with anti-infectives
Highlights of Prescribing Information
CIPRODEX® (ciprofloxacin and dexamethasone), otic suspension
Initial U.S. Approval: 2003
Indications and Usage for Ciprodex
CIPRODEX is a combination of ciprofloxacin, a fluoroquinolone antibacterial and dexamethasone, a corticosteroid, indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below:
- Acute Otitis Media (AOM) in pediatric patients (age 6 months and older) with tympanostomy tubes due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa. (1)
- Acute Otitis Externa (AOE) in pediatric (age 6 months and older), adult, and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa. (1)
Ciprodex Dosage and Administration
CIPRODEX is for otic use (ears) only, not for ophthalmic use, or for injection. (2.1)
- Shake well immediately before use. (2.1)
- Instill four drops into the affected ear twice daily, for seven days. (2.2)
Dosage Forms and Strengths
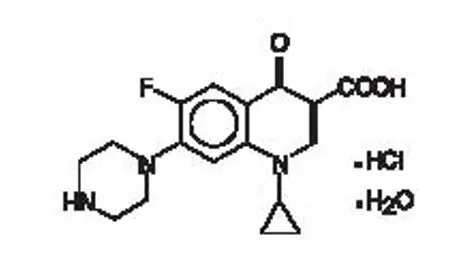
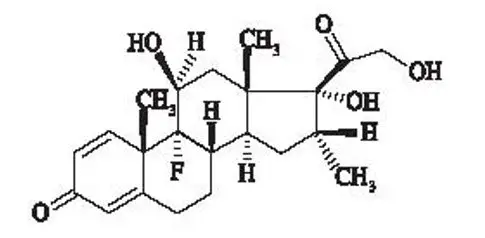
Otic Suspension: Each mL of CIPRODEX contains ciprofloxacin hydrochloride 0.3% (equivalent to 3 mg ciprofloxacin base) and dexamethasone 0.1% (equivalent to 1 mg dexamethasone). (3)
Contraindications
- CIPRODEX is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this medication. (4)
- Use of this product is contraindicated in viral infections of the external canal, including herpes simplex infections and fungal otic infections. (4)
Warnings and Precautions
- Hypersensitivity and anaphylaxis have been reported with systemic use of quinolones. Discontinue use if this occurs with use of CIPRODEX. (5.1)
- Prolonged use may result in overgrowth of non-susceptible bacteria and fungi. (5.2)
Adverse Reactions/Side Effects
Most common adverse reactions were ear discomfort (3%), ear pain (2.3%), and ear pruritus (1.5%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2020
Related/similar drugs
amoxicillin, cephalexin, azithromycin, Augmentin, ceftriaxone, amoxicillin / clavulanate, Cortisporin OticFull Prescribing Information
1. Indications and Usage for Ciprodex
CIPRODEX® is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below:
- Acute Otitis Media (AOM) in pediatric patients (age 6 months and older) with tympanostomy tubes due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa.
- Acute Otitis Externa (AOE) in pediatric (age 6 months and older), adult and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa.
2. Ciprodex Dosage and Administration
2.1 Important Administration Instructions
- CIPRODEX is for otic use (ears) only, and not for ophthalmic use, or for injection.
- Shake well immediately before use.
2.2 Dosage
For the Treatment of Acute Otitis Media in Pediatric Patients (age 6 months and older) With Tympanostomy Tubes
The recommended dosage regimen through tympanostomy tubes is as follows:
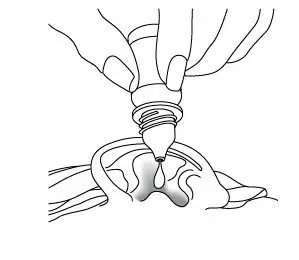
- Four drops [equivalent to 0.14 mL of CIPRODEX, (consisting of 0.42 mg of ciprofloxacin and 0.14 mg of dexamethasone)] instilled into the affected ear twice daily for seven days.
- The suspension should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness, which may result from the instillation of a cold suspension.
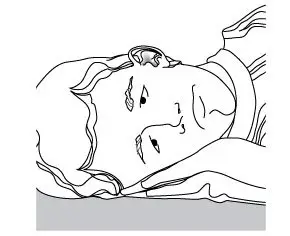
- The patient should lie with the affected ear upward, and then the drops should be instilled.
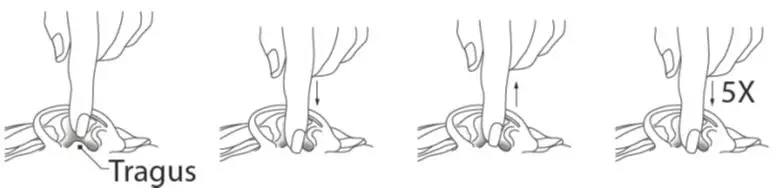
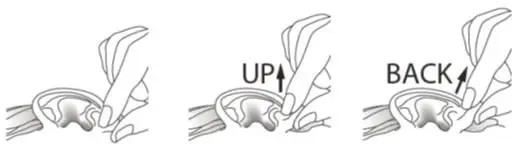
- The tragus should then be pumped 5 times by pushing inward to facilitate penetration of the drops into the middle ear.
- This position should be maintained for 60 seconds. Repeat, if necessary, for the opposite ear.
- Discard unused portion after therapy is completed.
For the Treatment of Acute Otitis Externa (age 6 months and older)
The recommended dosage regimen is as follows:
- Four drops [equivalent to 0.14 mL of CIPRODEX, (consisting of 0.42 mg ciprofloxacin and 0.14 mg dexamethasone)] instilled into the affected ear twice daily for seven days.
- The suspension should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness, which may result from the instillation of a cold suspension.
- The patient should lie with the affected ear upward, and then the drops should be instilled.
- This position should be maintained for 60 seconds to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
- Discard unused portion after therapy is completed.
3. Dosage Forms and Strengths
Otic Suspension: Each mL of CIPRODEX contains ciprofloxacin hydrochloride 0.3% (equivalent to 3 mg ciprofloxacin base) and dexamethasone 0.1% equivalent to 1 mg dexamethasone.
4. Contraindications
- CIPRODEX is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this medication.
- Use of this product is contraindicated in viral infections of the external canal, including herpes simplex infections and fungal otic infections.
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
CIPRODEX should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal, or facial edema), airway obstruction, dyspnea, urticaria, and itching.
5.2 Potential for Microbial Overgrowth with Prolonged Use
Prolonged use of CIPRODEX may result in overgrowth of non-susceptible bacteria and fungi. If the infection is not improved after one week of treatment, cultures should be obtained to guide further treatment. If such infections occur, discontinue use and institute alternative therapy.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Potential for Microbial Overgrowth with Prolonged Use [see Warnings and Precautions (5.2)]
| CIPRODEX
ciprofloxacin and dexamethasone suspension/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |