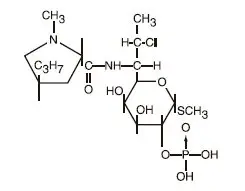
Drug Detail:Clindacin etz (Clindamycin topical [ klin-da-mye-sin-top-ik-al ])
Drug Class: Topical acne agents
Contraindications
Clindacin® ETZ is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional enteritis or ulcerative colitis, or a history of antibiotic-associated colitis.
Warnings
Orally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption of the antibiotic from the skin surface. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin.
Studies indicate a toxin(s) produced by clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Endoscopic examination may reveal pseudomembranous colitis. Stool culture for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
When significant diarrhea occurs, the drug should be discontinued. Large bowel endoscopy should be considered to establish a definitive diagnosis in cases of severe diarrhea.
Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen the condition. Vancomycin has been found to be effective in the treatment of antibiotic-associated pseudomembranous colitis produced by Clostridium difficile. The usual adult dosage is 500 milligrams to 2 grams of vancomycin orally per day in three to four divided doses administered for 7 to 10 days. Cholestyramine or colestipol resins bind vancomycin in vitro. If both a resin and vancomycin are to be administered concurrently, it may be advisable to separate the time of administration of each drug.
Diarrhea, colitis, and pseudomembranous colitis have been observed to begin up to several weeks following cessation of oral and parenteral therapy with clindamycin.
Adverse Reactions/Side Effects
In 18 clinical studies of various formulations of topical clindamycin phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent adverse dermatologic events [see table below].
| Number of Patients Reporting Events | |||
|---|---|---|---|
| Treatment Emergent Adverse Event | Solution n=553 (%) | Gel n=148 (%) | Lotion n=160 (%) |
|
|||
| Burning | 62 (11) | 15 (10) | 17 (11) |
| Itching | 36 (7) | 15 (10) | 17 (11) |
| Burning/Itching | 60 (11) | * (-) | * (-) |
| Dryness | 105 (19) | 34 (23) | 29 (18) |
| Erythema | 86 (16) | 10 (7) | 22 (14) |
| Oiliness/Oily Skin | 8 (1) | 26 (18) | 12† (10) |
| Peeling | 61 (11) | * (-) | 11 (7) |
Orally and parenterally administered clindamycin has been associated with severe colitis which may end fatally. Cases of diarrhea, bloody diarrhea and colitis (including pseudomembranous colitis) have been reported as adverse reactions in patients treated with oral and parenteral formulations of clindamycin and rarely with topical clindamycin (see WARNINGS).
Abdominal pain, gastrointestinal disturbances, gram-negative folliculitis, eye pain and contact dermatitis have also been reported in association with the use of topical formulations of clindamycin.
| CLINDACIN ETZ
clindamycin phosphate swab |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CLINDACIN ETZ
clindamycin phosphate kit |
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
| Labeler - Medimetriks Pharmaceuticals, Inc. (019903816) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Perrigo Inc. | 078846912 | MANUFACTURE(43538-172, 43538-173) | |