Drug Detail:Clindesse (Clindamycin vaginal cream (one-dose) [ klin-da-mye-sin ])
Drug Class: Vaginal anti-infectives
Highlights of Prescribing Information
Clindesse® (clindamycin phosphate) Vaginal Cream, 2%
For intravaginal use only.
Initial U.S. Approval: 1972
Indications and Usage for Clindesse
Clindesse is a lincosamide antibacterial indicated for the treatment of bacterial vaginosis in non-pregnant women (1.1)
Clindesse Dosage and Administration
- •
- A single applicator of cream administered once intravaginally at any time of the day (2)
- •
- Not for ophthalmic, dermal, or oral use
Dosage Forms and Strengths
One single-dose, pre-filled disposable applicator delivers approximately 5 g of cream containing approximately 100 mg of clindamycin (3)
Contraindications
- •
- History of hypersensitivity to clindamycin or other lincosamides (4.1)
- •
- History of regional enteritis, ulcerative colitis, or a history of C. difficile-associated diarrhea (4.2, 5.1)
Warnings and Precautions
- •
- C. difficile-associated diarrhea: Discontinue and evaluate if diarrhea occurs (5.1)
- •
- Rubber or latex products: Do not use barrier contraceptives (condoms or vaginal contraceptive diaphragms) concurrently or for 5 days following treatment. Condoms may not be reliable for preventing pregnancy or for protecting again transmission of HIV and other sexually transmitted diseases during this time (5.2)
Adverse Reactions/Side Effects
Most common adverse reactions reported in ≥2% of patients and at a higher rate in the Clindesse group than in the placebo group are vaginosis fungal (14%), headache (7%), back pain (5%), constipation (2%), and urinary tract infection (2%) (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Neuromuscular blocking agents: Enhanced action of neuromuscular blocking agents can occur; use with caution (7.1)
Use In Specific Populations
Nursing Mothers: Caution should be exercised when administered to a nursing woman (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2023
Related/similar drugs
metronidazole, clindamycin, Cleocin, tinidazoleFull Prescribing Information
2. Clindesse Dosage and Administration
The recommended dose is the complete contents of a single pre-filled applicator containing 5 g of Clindesse cream administered once intravaginally at any time of the day.
Not for ophthalmic, dermal, or oral use.
3. Dosage Forms and Strengths
Clindesse is an intravaginal cream containing clindamycin phosphate 2%. Each pre-filled, single-dose applicator delivers approximately 5 g of cream containing approximately 100 mg of clindamycin.
4. Contraindications
5. Warnings and Precautions
5.1 Clostridium difficile-Associated Diarrhea (CDAD)
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated [see Adverse Reactions (6.2].
5.2 Use with Condoms and Vaginal Contraceptive Diaphragms
This cream contains mineral oil that may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms. Therefore, the use of such barrier contraceptives is not recommended concurrently or for 5 days following treatment with Clindesse. During this time period, condoms may not be reliable for preventing pregnancy or for protecting against transmission of HIV and other sexually transmitted diseases.
6. Adverse Reactions/Side Effects
6.1 Clinical Study Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to Clindesse in 368 patients. Clindesse was studied in three clinical studies: placebo-controlled (n=85), active-controlled (n=263), and single-arm (n=20). The population was female, aged 18 to 78, who were diagnosed with bacterial vaginosis. Patient demographics in the trials were 51% Caucasian, 36% Black, 10% Hispanic, and 3% Asian, other or unknown. All patients received 100 mg clindamycin phosphate cream intravaginally in a single dose.
Of the 368 women treated with a single dose of Clindesse, 1.6% of the patients discontinued therapy due to adverse reactions. Adverse reactions occurred in 126 of 368 patients (34%) treated with Clindesse and in 32 of 85 patients (38%) treated with placebo.
Adverse reactions occurring in ≥2% of patients receiving Clindesse in the placebo-controlled clinical trial are shown in Table 1.
Table 1. Adverse Reactions Occurring in ≥2% of Clindesse-Treated Patients and at a Higher Rate than Placebo-Treated Patients
N = number of patients in intent-to-treat population
n (%) = number and percentage of patients with reported adverse reaction
NOS = not otherwise specified
- The use of clindamycin may result in the overgrowth of non-susceptible fungal organisms in the vagina and may require antifungal treatment
- Other reactions reported by <1% of those women treated with Clindesse include:
- Dermatologic: Pruritic rash
- Gastrointestinal: Diarrhea, vomiting
- General: Fatigue
- Immune System: Hypersensitivity
- Nervous System: Dizziness
- Reproductive System: Dysfunctional uterine bleeding, dysmennorrhea, intermenstrual bleeding, pelvic pain, vaginal burning, vaginal irritation, vulvar erythema, vulvitis, vulvovaginal discomfort, vulvovaginal dryness, vulvovaginitis
6.2 Other Clindamycin Formulations
Clindesse affords minimal peak serum levels and systemic exposure (AUCs) of clindamycin compared to an oral or intravenous dose of clindamycin [see Clinical Pharmacology (12.1)]. Data from well-controlled trials directly comparing clindamycin administered orally to clindamycin administered vaginally are not available.
The following additional adverse reactions and altered laboratory tests have been reported with the oral or parenteral use of clindamycin:
Gastrointestinal: Abdominal pain, esophagitis, nausea, Clostridium difficile-associated diarrhea [see Warnings and Precautions (5.1)].
Hematopoietic: Transient neutropenia (leukopenia), eosinophilia, agranulocytosis, and thrombocytopenia have been reported. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of these reports.
Hypersensitivity Reactions: Maculopapular rash, vesiculobullous rash, and urticaria have been observed during drug therapy. Generalized mild to moderate morbilliform-like skin rashes are the most frequently reported of all adverse reactions. Cases of erythema multiforme, some resembling Stevens-Johnson syndrome, have been associated with clindamycin. A few cases of anaphylactoid reactions have been reported.
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Musculoskeletal: Cases of polyarthritis have been reported.
Renal: Although no direct relationship of clindamycin to renal damage has been established, renal dysfunction as evidenced by azotemia, oliguria, and/or proteinuria has been observed in rare instances.
Immune System: Drug reaction with eosinophilia and systemic symptoms (DRESS) cases have been reported.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Clindesse. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic: Rash
Gastrointestinal: Hematochezia
Reproductive System: Vaginal erythema, vulvovaginal pruritis, vaginal discharge, vaginal swelling, vaginal bleeding, vaginal pain
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Category B
Clindesse should be used during pregnancy only if clearly needed. There are no adequate and well-controlled studies of Clindesse in pregnant women.
Another intravaginal formulation containing 2% clindamycin phosphate has been studied in pregnant women during the second trimester. In women treated for seven days, abnormal labor was reported in 1.1% of patients who received that clindamycin vaginal cream formulation compared with 0.5% of patients who received placebo.
Reproduction studies have been performed in rats and mice using oral and parenteral doses of clindamycin up to 600/mg/kg/day (58 and 29 times, respectively, the recommended human dose based on body surface area comparisons) and have revealed no evidence of harm to the fetus due to clindamycin.
Because animal reproduction studies are not always predictive of human response, Clindesse should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
Caution should be exercised when Clindesse is administered to a nursing woman. It is not known if clindamycin is excreted in human milk following the use of vaginally administered clindamycin. Clindamycin has been detected in human milk after oral or parenteral administration.
Because of the potential for serious adverse reactions in nursing infants, a decision to continue or discontinue nursing should take into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and efficacy of Clindesse in the treatment of bacterial vaginosis in post-menarchal females have been established on the extrapolation of clinical trial data from adult women. The safety and efficacy of Clindesse in pre-menarchal females have not been established.
8.5 Geriatric Use
Clinical studies with Clindesse did not include sufficient numbers of subjects 65 years of age or older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
10. Overdosage
Vaginally applied clindamycin phosphate vaginal cream 2% could be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)].
11. Clindesse Description
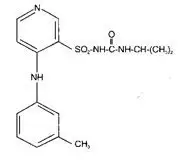
Clindamycin phosphate, a lincosamide, is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin. The chemical name for clindamycin phosphate is methyl 7-chloro- 6,7,8-trideoxy-6-(1-methyl- trans- 4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L- threo-(alpha)-D-galacto- octopyranoside 2-(dihydrogen phosphate). It has a molecular weight of 504.95, and the molecular formula is C18H34CIN2O8PS. The structural formula is represented below:
Clindesse is a semi-solid, white cream, which contains clindamycin phosphate, USP, at a concentration equivalent to 20 mg clindamycin base per gram. The cream also contains edetate disodium, glycerol monoisostearate, lecithin, methylparaben, microcrystalline wax, mineral oil, polyglyceryl-3-oleate, propylparaben, purified water, silicon dioxide and sorbitol solution.
Clindesse does not comply with the pH test of the USP monograph for clindamycin phosphate vaginal cream.
12. Clindesse - Clinical Pharmacology
12.1 Mechanism of Action
Clindamycin is an antibacterial drug [see Clinical Pharmacology, Microbiology (12.4)].
12.3 Pharmacokinetics
Following a single intravaginal application of Clindesse cream to 20 healthy women, the mean (range) AUC0-inf and Cmax estimates were 175 (38.6 to 541) ng/mL●hr and 6.6 (0.8 to 39) ng/mL, respectively. The mean Cmaxof clindamycin for Clindesse was approximately 0.3%, 0.1%, and 7.6% of that observed after the administration of a 150 mg Cleocin oral capsule (2.5 mcg/mL), a 600 mg Cleocin intravenous injection (10.9 mcg/mL), and a single dose of 100 mg of Cleocin Vaginal Cream (86.5 ng/mL), respectively. The peak serum concentration of clindamycin was attained approximately 20 hours post dosing for Clindesse.
12.4 Microbiology
Mechanism of Action
Clindamycin inhibits bacterial protein synthesis at the level of the bacterial ribosome. The antibiotic binds preferentially to the 50S ribosomal subunit and affects the process of peptide chain initiation. Although clindamycin phosphate is inactive in vitro, in vivo hydrolysis converts this compound to the antibacterially active clindamycin.
Activity In Vitro
Clindamycin is an antibacterial agent active in vitro against most strains of the following organisms that have been reported to be associated with bacterial vaginosis:
Bacteroidesspp.
Gardnerellavaginalis
Mobiluncusspp.
Mycoplasma hominis
Peptostreptococcusspp.
Standard methodology for the susceptibility testing of the potential bacterial vaginosis pathogens has not been defined. Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of bacterial vaginosis [see Clinical Studies (14)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames test. Both tests were negative. Fertility studies in rats treated orally with up to 300/mg/kg/day (29 times the recommended human dose based on body surface area comparisons) revealed no effects on fertility or mating ability.
14. Clinical Studies
Two clinical studies were conducted to evaluate the efficacy of Clindesse for the treatment of bacterial vaginosis. A clinical diagnosis of bacterial vaginosis was defined by the presence of a homogeneous vaginal discharge that (a) has a pH of greater than 4.5, (b) emits a “fishy” amine odor when mixed with a 10% KOH solution, and (c) contains clue cells on microscopic examination. Gram’s stain results consistent with a diagnosis of bacterial vaginosis include (a) markedly reduced or absent Lactobacillus morphology, (b) predominance of Gardnerellamorphotype, and (c) absent or few white blood cells.
In a randomized, double-blind, placebo-controlled, clinical study involving 144 non-pregnant female patients aged 18 to 64 with a baseline Nugent score ≥4, Clindesse demonstrated statistically significantly higher cure rates over placebo as measured by therapeutic cure, clinical cure, and Nugent score cure (Table 2) assessed at 21-30 days after administration of the drug. Therapeutic cure was a composite endpoint which required both clinical cure and Nugent score cure. Clinical cure required normal vaginal discharge, vaginal pH < 4.7, < 20% clue cells on wet mount preparation, and negative “whiff” test (detection of amine odor on addition of 10% KOH solution to sample of the vaginal discharge). A Nugent score of 0-3 was considered a Nugent score cure. The Nugent scoring is based on microscopic examination of the Gram’s stained vaginal smears for quantification of specific bacterial morphotypes. Cure rates were consistently higher for Clindesse compared to placebo for the following demographic subsets: age, race, height, weight, sexual behavior, and recalcitrant infection status.
Table 2. Efficacy of Clindesse for Treatment of Bacterial Vaginosis in a Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study
N = number of patients in treatment group (modified intent-to-treat population defined as all subjects randomized who received at least one dose of study medication, and who had a baseline Nugent score of at least 4)
†Treatment difference = Clindesse minus placebo cure rates
In a second controlled clinical study involving 432 patients aged 18 to 78 with a baseline Nugent score of ≥4, 221 women self-administered a single dose of Clindesse, and 211 women self-administered a single daily dose of a formulation of clindamycin vaginal cream for 7 days. A single dose of Clindesse was shown to be similar to 7 daily doses of the clindamycin vaginal cream for treatment of bacterial vaginosis as measured by therapeutic cure, clinical cure of Nugent score cure assessed at 21-30 days after administration of the drug in the modified intent-to-treat population (Table 3) and for the per protocol population (Table 4). The study endpoints were identical to those described above for the placebo-controlled study. Statistical analyses did not reveal any significant differences when controlling for the following demographic variables: age, race, height, weight, sexual behavior, and recalcitrant infection status.
The cure rates reported in the clinical studies with Clindesse were based on resolution of 4 out of 4 Amsel criteria and a Nugent score of < 4, while the criteria for cure in previous clinical studies with the clindamycin vaginal cream were based solely on resolution of 2 out of 4 Amsel criteria, resulting in higher reported rates of cure for bacterial vaginosis.
Table 3. Efficacy of Clindesse in Treatment of Bacterial Vaginosis in a Randomized, Investigator-Blind, Active-Controlled Comparative Study – Modified-Intent-to-Treat
†Treatment difference = Clindesse minus clindamycin vaginal cream cure rates
N = number of patients in treatment group (modified intent-to-treat population defined as all subjects randomized who received at least one dose of study medication, and who had a baseline Nugent score of at least 4)
Table 4. Efficacy of Clindesse in Treatment of Bacterial Vaginosis in a Randomized, Investigator-Blind, Active-Controlled Comparative Study – Per Protocol
†Treatment difference = Clindesse minus clindamycin vaginal cream cure rates
N = number of patients in treatment group (per protocol population defined as all subjects included in the modified intent-to-treat population who completed the study without significant protocol violation)
‡Four subject (2 from each treatment group) did not have complete Nugent scores and were not included in the Nugent Score cure analysis
16. How is Clindesse supplied
Clindesse (clindamycin phosphate) Vaginal Cream, 2%, is available in cartons containing one single-dose, pre-filled disposable applicator (NDC 45802-042-01). Each applicator delivers approximately 5 g of vaginal cream containing approximately 100 mg of clindamycin.
Store at 20°-25°C (68°-77°F [See USP Controlled Room Temperature.]
Avoid heat above 30°C (86°F).
17. Patient Counseling Information
17.1 Vaginal Intercourse and Use with Vaginal Products
Instruct the patient not to engage in vaginal intercourse, or use other vaginal products (such as tampons or douches) during treatment with this product.
17.2 Use with Condoms and Vaginal Contraceptive Diaphragms
Advise the patient that this cream contains mineral oil that may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms. Therefore, do not use barrier contraceptives concurrently or for 5 days following treatment with Clindesse. During this time period, condoms may not be reliable for preventing pregnancy or for protecting against transmission of HIV and other sexually transmitted diseases [see Warnings and Precautions (5.2)].
17.3 Fungal Vaginal Infections
Inform the patient that vaginal fungal infections can occur following use of Clindesse and may require treatment with an antifungal drug [see Adverse Reactions (6.1)].
17.4 Accidental Exposure to the Eye
Inform the patient the Clindesse contains ingredients which cause burning and irritation of the eye. In the event of accidental contact with the eye, rinse the eye with copious amounts of cool tap water and consult a physician.
Manufactured by Padagis®
Yeruham, Israel
www.padagis.com
Rev 05-23
2S000 RC PH3
Patents at www.padagis.com/patents
Cleocin is a registered trademark of Pharmacia & Upjohn Company.
Patient Information
Patient Information
Clindesse (clin-DESS)
(clindamycin phosphate)
Vaginal Cream, 2%
For vaginal use only.
Do not put Clindesse in your eyes, mouth, or on your skin.
Read this patient information before you start using Clindesse. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is Clindesse?
Clindesse is a vaginal cream medicine used to treat bacterial vaginal infections in women who are not pregnant.
It is not known if Clindesse is safe and effective in pregnant women.
It not known if Clindesse is safe and effective in females who have not yet reached puberty.
Who should not use Clindesse?
Do not use Clindesse if you:
- •
- have had an allergic reaction to clindamycin or other lincosamide antibiotic medicines or are allergic to any of the ingredients in Clindesse. See the end of this leaflet for a complete list of ingredients in Clindesse.
- •
- had had bowel problems such as:
- ∘
- inflammation of your intestines (enteritis)
- ∘
- inflammation of your colon (colitis)
- ∘
- diarrhea due to a Clostridum difficile infection (CDAD)
Talk to your healthcare provider before using this medicine if you have any of these conditions.
What should I tell my healthcare provider before using Clindesse?
Before you use Clindesse, tell your healthcare provider if you:
- •
- are pregnant or plan to become pregnant. It is not known if Clindesse will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if Clindesse passes into your breast milk. You and your healthcare provider should decide if you will take Clindesse or breast feed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Clindesse may affect how other medicines work, and other medicines may affect how Clindesse works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Clindesse?
- •
- Use Clindesse exactly as your healthcare provider tells you.
- •
- Insert Clindesse in the vagina one time. See the “Patient Instructions for Use” at the end of this leaflet.
- •
- Insert all of the Clindesse cream into your vagina.
- •
- Do not use Clindesse after the expiration date on the package.
- •
- Do not get Clindesse in your eyes. If you accidently get Clindesse in your eyes rinse your eyes with cool tap water right away and call your healthcare provider.
What should I avoid while using Clindesse?
After you insert Clindesse you should:
- •
- not have vaginal intercourse or use of other vaginal products (such as tampons or douches) for at least 7 days.
- •
- not use barrier contraceptive products for 5 days. Barrier contraceptives include condoms or contraceptive diaphragms used for birth control or to protect yourself against HIV or other sexually transmitted diseases. Clindesse contains mineral oil that may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms.
What are the possible side effects of Clindesse?
Clindesse may cause serious side effects, including diarrhea. One type of diarrhea is caused by an infection in your intestines called Clostridium difficile-associated diarrhea (CDAD). If you have diarrhea after you use Clindesse, call your healthcare provider.
The most common side effects of Clindesse include:
- •
- fungal infection in your vagina. You may need to take an anti-fungal medicine if you get a fungal infection.
- •
- headache
- •
- back pain
- •
- constipation
- •
- urinary tract infection
Tell your healthcare provider if you have any side effect that bothers you or that does not away.
These are not all of the possible side effects of Clindesse. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Clindesse?
- •
- Store Clindesse at 68° to 77°F (20° to 25°).
- •
- Keep Clindesse and all medicines out of the reach of children.
General information about the safe and effective use of Clindesse.
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not give Clindesse to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Clindesse. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information Clindesse that is written for health professionals.
For more information, go to www.clindesse.com or call 1-866-634-9120
What are the ingredients in Clindesse?
Active ingredient: clindamycin phosphate
Inactive ingredients: edetate disodium, glycerol monoisostearate, lecithin, methylparaben, microcrystalline wax, mineral oil, polyglyceryl-3-oleate, propylparaben, purified water, silicon dioxide and sorbitol solution
Patient Instructions for Use
For vaginal use only.
Do not put Clindesse in your eyes, mouth, or on your skin.
It is important that you read and follow these directions on how to use Clindesse vaginal cream properly.
Clindesse comes in a single-dose, pre-filled, disposable applicator that gives you a certain amount of clindamycin cream to be inserted into your vagina.
Step 1. Prepare the applicator.
- •
- Peel back the protective foil and remove the pre-filled applicator. Do not remove the tip. The applicator is made to be used with the tip in place. Do not use the applicator if the tip has been removed (see Figure 1).
- •
- Activate the plunger before you use it. To activate the plunger, pull the ring back to fully extend the plunger while you firmly hold the applicator (see Figure 2).
Step 2. Insert the applicator.
- •
- Gently insert the applicator into your vagina as far as it will comfortably go (see Figure 3).
Step 3. Apply the cream.
Push the plunger in until all of the cream goes into your vagina (see Figures 4 and 5).
Step 4. Remove the empty applicator from your vagina and throw it away in the trash.
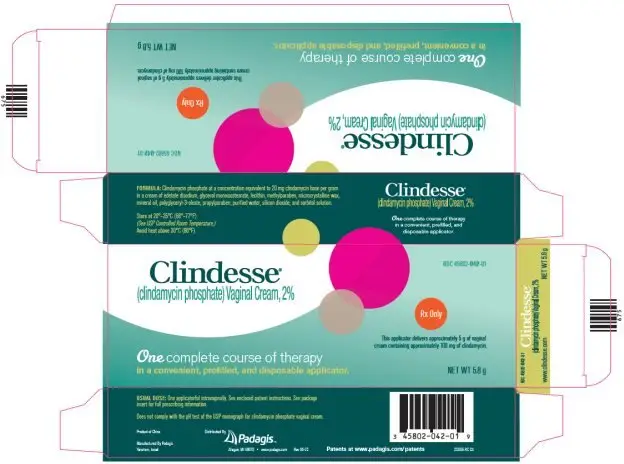
Package/Label Display Panel - Carton
Clindesse®
(clindamycin phosphate) Vaginal Cream, 2%
Rx Only
This applicator delivers approximately 5 g of vaginal cream containing approximately 100 mg of clindamycin.
One complete course of therapy in a convenient, prefilled, and disposable applicator.
NET WT 5.8 g
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
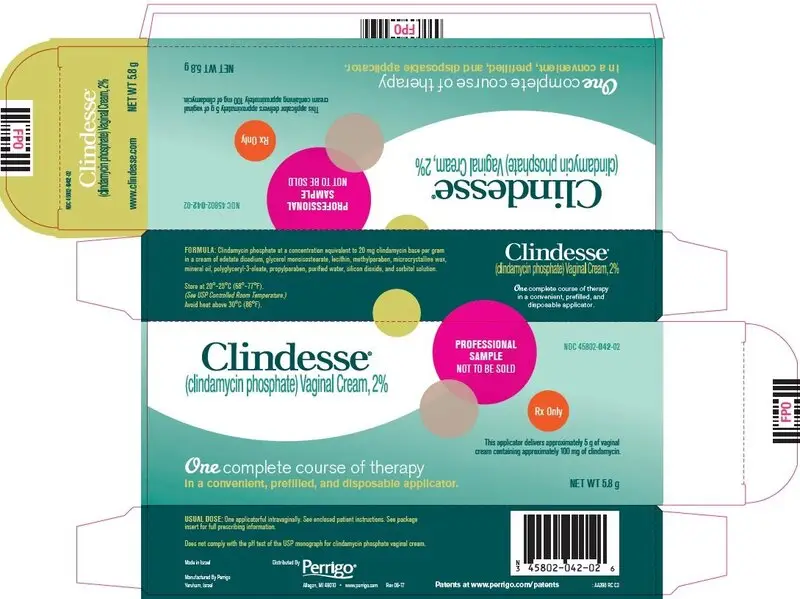
PACKAGE/LABEL DISPLAY PANEL – Professional Sample – Not to be Sold
Clindesse®
(clindamycin phosphate) Vaginal Cream, 2%
PROFESSIONAL SAMPLE NOT TO BE SOLD
Rx Only
This applicator delivers approximately 5 g of vaginal cream containing approximately 100 mg of clindamycin.
One complete course of therapy in a convenient, prefilled, and disposable applicator.
NET WT 5.8 g
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
| CLINDESSE
clindamycin phosphate cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Padagis Israel Pharmaceuticals Ltd (600093611) |