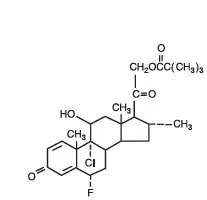
Drug Detail:Cloderm cream (Clocortolone pivalate 0.1%)
Drug Class: Topical steroids
Precautions
Pediatric Use
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area body weight ratio.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
Cloderm Dosage and Administration
Apply Cloderm (clocortolone pivalate) Cream 0.1% sparingly to the affected areas three times a day and rub in gently.
Occlusive dressings may be used for the management of psoriasis or recalcitrant conditions.
If an infection develops, the use of occlusive dressings should be discontinued and appropriate anti-microbial therapy instituted.
| CLODERM
clocortolone pivalate cream |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - EPI Health, Inc (080638894) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EPI Health, Inc. | 080638894 | MANUFACTURE(71403-804) | |