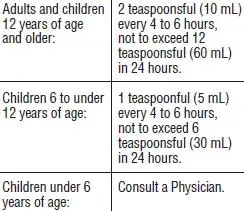
Drug Detail:Codar gf (Codeine and guaifenesin [ koe-deen-and-gwye-fen-a-sin ])
Drug Class: Upper respiratory combinations
| CODEINE PHOSPHATE GUAIFENESIN
codeine phosphate, guaifenesin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kylemore Pharmaceuticals, LLC (831892471) |