Drug Detail:Combivent respimat (Albuterol and ipratropium [ al-bue-ter-ol-and-ip-ra-tro-pee-um ])
Drug Class: Bronchodilator combinations
Combivent Description
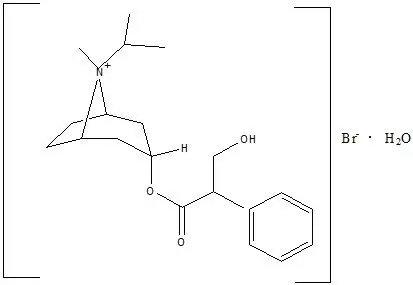
C20H30BrNO3•H2O ipratropium bromide Mol. Wt. 430.4
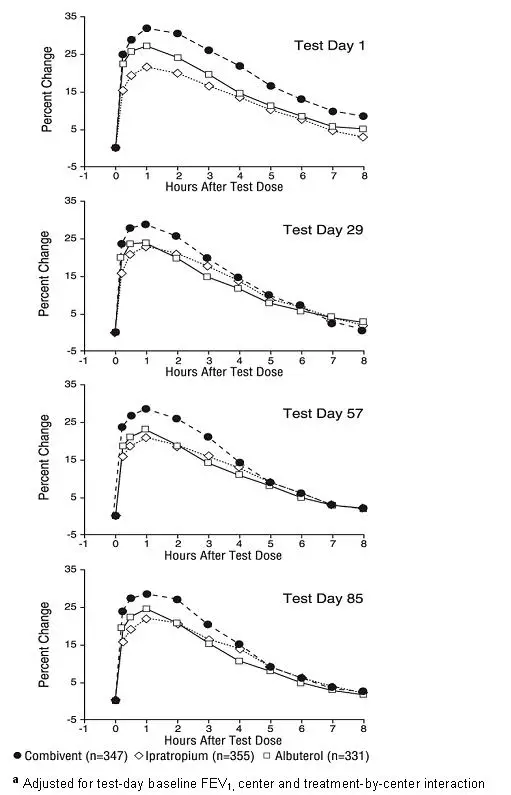
Combivent - Clinical Pharmacology
Drug-Drug Interactions
No specific pharmacokinetic studies were conducted to evaluate potential drug-drug interactions.
Precautions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Adverse Reactions/Side Effects
| COMBIVENT Ipratropium Bromide 36 mcg/Albuterol Sulfate 206 mcg QID | Ipratropium Bromide 36 mcg QID | Albuterol Sulfate 206 mcg QID |
|
|---|---|---|---|
| N = 358 | N = 362 | N = 347 | |
| *All adverse events, regardless of drug relationship, reported by two percent or more patients in one or more treatment group in the 12-week controlled clinical trials. | |||
| Body as a Whole-General Disorders | |||
| Headache | 5.6 | 3.9 | 6.6 |
| Pain | 2.5 | 1.9 | 1.2 |
| Influenza | 1.4 | 2.2 | 2.9 |
| Chest Pain | 0.3 | 1.4 | 2.9 |
| Gastrointestinal System Disorders | |||
| Nausea | 2.0 | 2.5 | 2.6 |
| Respiratory System Disorders (Lower) | |||
| Bronchitis | 12.3 | 12.4 | 17.9 |
| Dyspnea | 4.5 | 3.9 | 4.0 |
| Coughing | 4.2 | 2.8 | 2.6 |
| Respiratory Disorders | 2.5 | 1.7 | 2.3 |
| Pneumonia | 1.4 | 2.5 | 0.6 |
| Bronchospasm | 0.3 | 3.9 | 1.7 |
| Respiratory System Disorders (Upper) | |||
| Upper Respiratory Tract Infection | 10.9 | 12.7 | 13.0 |
| Pharyngitis | 2.2 | 3.3 | 2.3 |
| Sinusitis | 2.3 | 1.9 | 0.9 |
| Rhinitis | 1.1 | 2.5 | 2.3 |
How is Combivent supplied
Each 14.7 gram canister provides sufficient medication for 200 actuations (NDC 0597-0013-14).
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Ipratropium bromide licensed from:
Boehringer Ingelheim International GmbH
Copyright 2012 Boehringer Ingelheim Pharmaceuticals, Inc.
ALL RIGHTS RESERVED
Patient's Instructions for Use
Combivent®
(ipratropium bromide and albuterol sulfate)
Inhalation Aerosol
Read complete instructions carefully before using
-
Insert metal canister into clear end of mouthpiece (see Figure 1). Make sure the canister is fully and firmly inserted into the mouthpiece. The COMBIVENT Inhalation Aerosol canister is to be used only with the COMBIVENT Inhalation Aerosol mouthpiece. This mouthpiece should not be used with other inhaled medicines.
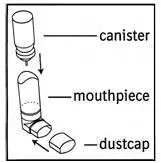
Figure 1
-
Remove orange protective dust cap. If the cap is not on the mouthpiece, make sure there is nothing in the mouthpiece before use. For best results, the canister should be at room temperature before use.
-
Shake and Test Spray. Perform this step before using for the first time, and whenever the aerosol has not been used for more than 24 hours; otherwise, proceed directly to Step 4.
-
Shake the canister vigorously for at least 10 seconds. Hold canister as illustrated in Figure 2.
For best results, perform Steps 5 and 6 within 30 seconds of shaking the canister.
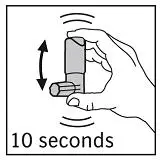
Figure 2
-
Breathe out (exhale) deeply through your mouth. Holding the canister upright as shown in Figure 3, between your thumb and finger(s), put the mouthpiece in your mouth and close your lips. Keep your eyes closed so that no medicine will be sprayed into your eyes. Combivent® (ipratropium bromide and albuterol sulfate) Inhalation Aerosol can cause blurry vision, narrow-angle glaucoma or worsening of this condition or eye pain if the medicine is sprayed into your eyes.
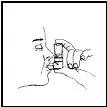
Figure 3
-
Breathe in (inhale) slowly through your mouth and at the same time spray the product into your mouth.
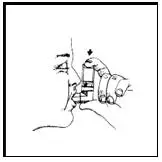
Figure 4
-
Hold your breath for 10 seconds, remove the mouthpiece from your mouth and breathe out slowly, as in Figure 5.
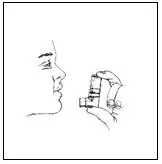
Figure 5
-
Wait approximately 2 minutes, shake the inhaler vigorously for at least 10 seconds again (as described in Step 4), and repeat Steps 5 to 7.
-
Replace the orange protective dust cap after use.
-
Keep the mouthpiece clean. Wash with hot water. If soap is used, rinse thoroughly with plain water. Dry thoroughly before use. When dry, replace cap on the mouthpiece when not using the drug product.
-
Keep track of the number of sprays used and discard after 200 sprays. Even though the canister is not empty, you cannot be sure of the amount of medicine in each spray after 200 sprays.
- If your prescribed dose does not provide relief or your breathing symptoms become worse, get medical help right away.
- This product contains trichloromonofluoromethane (CFC-11), dichlorodifluoromethane (CFC-12) and dichlorotetrafluoroethane (CFC-114), substances which harm the environment by destroying ozone in the upper atmosphere.
Keep COMBIVENT Inhalation Aerosol out of reach of children.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Ipratropium bromide licensed from:
Boehringer Ingelheim International GmbH
Copyright 2012 Boehringer Ingelheim Pharmaceuticals, Inc.
ALL RIGHTS RESERVED
| COMBIVENT
ipratropium bromide and albuterol sulfate aerosol, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| 3M Drug Delivery Systems | 128688199 | MANUFACTURE(0597-0013) , ANALYSIS(0597-0013) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | ANALYSIS(0597-0013) , API MANUFACTURE(0597-0013) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Roxane Inc. | 058839929 | ANALYSIS(0597-0013) , PACK(0597-0013) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Roxane, Inc. | 007074234 | MANUFACTURE(0597-0013) , ANALYSIS(0597-0013) , PACK(0597-0013) | |