Drug Class: Bronchodilator combinations
Highlights of Prescribing Information
COMBIVENT® RESPIMAT® (ipratropium bromide and albuterol inhalation spray), for oral inhalation use
Initial U.S. Approval: 1996
Indications and Usage for Combivent Respimat
COMBIVENT RESPIMAT Inhalation Spray is a combination of ipratropium bromide (an anticholinergic agent) and albuterol sulfate (a beta2‑adrenergic agonist) indicated for:
- Patients with chronic obstructive pulmonary disease (COPD) on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator (1)
Combivent Respimat Dosage and Administration
For oral inhalation only
- One inhalation four times a day, not to exceed six inhalations in 24 hours (2)
Dosage Forms and Strengths
- Inhalation spray: 20 mcg ipratropium bromide (monohydrate) and 100 mcg albuterol (equivalent to 120 mcg albuterol sulfate) per actuation with the COMBIVENT RESPIMAT inhaler (3)
Contraindications
- Hypersensitivity to any of the ingredients in COMBIVENT RESPIMAT (4)
- Hypersensitivity to atropine or any of its derivatives (4)
Warnings and Precautions
- Paradoxical bronchospasm: Discontinue COMBIVENT RESPIMAT immediately and treat with alternative therapy if paradoxical bronchospasm occurs (5.1)
- Patients with cardiovascular system disorders: Use with caution because of beta-adrenergic stimulation (5.2)
- Ocular effects: Advise patients to avoid spraying into eyes and to contact a physician if blurred vision, halos, or other visual disturbances occur. Monitor patients with narrow-angle glaucoma. (5.3)
- Urinary retention: Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction (5.4)
- Hypersensitivity reactions including anaphylaxis: Discontinue COMBIVENT RESPIMAT and institute alternative therapy if immediate hypersensitivity reactions such as urticaria, angioedema, rash, bronchospasm, anaphylaxis, or oropharyngeal edema occur (5.6)
- Coexisting conditions: Use with caution in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus (5.7)
Adverse Reactions/Side Effects
Most common (≥2%) adverse reactions for COMBIVENT RESPIMAT (20/100 mcg) are upper respiratory infection, nasopharyngitis, cough, bronchitis, headache, and dyspnea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of COMBIVENT RESPIMAT with other anticholinergic-containing drugs (7.1)
- Beta-adrenergic agonists: May increase the risk of adverse cardiovascular effects. Avoid coadministration of COMBIVENT RESPIMAT and other sympathomimetic agents (7.2)
- Beta-blockers: Inhibit the effect of albuterol. Consider alternative therapy in patients with hyperreactive airways (7.3)
- Diuretics: Electrocardiographic changes and/or hypokalemia associated with diuretics may worsen with concomitant use of beta-agonists. Consider monitoring potassium levels. (7.4)
- Monoamine oxidase inhibitors (MAOs) or tricyclic antidepressants: May potentiate effect of albuterol on the vascular system. Consider alternative therapy in patients taking MAOs or tricyclic antidepressants. (7.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2021
Full Prescribing Information
Contraindications
- Hypersensitivity to any of the ingredients in COMBIVENT RESPIMAT
- Hypersensitivity to atropine or any of its derivatives
Warnings and Precautions
Adverse Reactions/Side Effects
Use of albuterol, a beta2-adrenergic agonist, may be associated with the following:
- Paradoxical bronchospasm [see Warnings and Precautions (5.1)]
- Cardiovascular effects [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions including anaphylaxis [see Contraindications (4) and Warnings and Precautions (5.6)]
- Hypokalemia [see Warnings and Precautions (5.8)]
Albuterol is a component of COMBIVENT RESPIMAT.
Use of ipratropium bromide, an anticholinergic, may result in the following:
- Ocular effects [see Warnings and Precautions (5.3)]
- Urinary retention [see Warnings and Precautions (5.4)]
Ipratropium bromide is a component of COMBIVENT RESPIMAT.
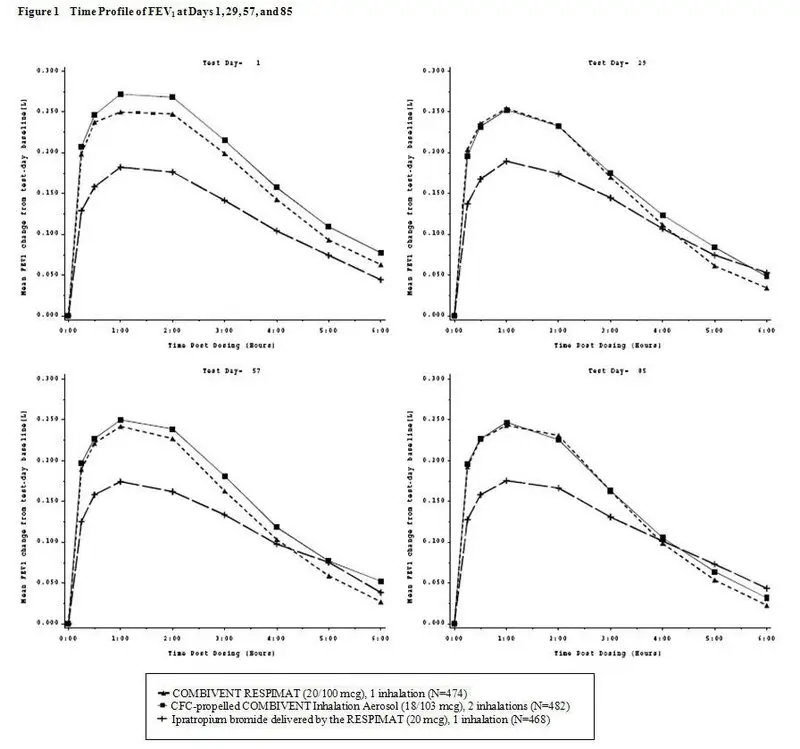
6.1 Clinical Trials Experience
COMBIVENT RESPIMAT 12-Week Clinical Trials
| Body System (Event) | 12-Week Ipratropium-Controlled Trial |
|||
| COMBIVENT RESPIMAT (20/100 mcg) | CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg) | Ipratropium bromide by the RESPIMAT Inhaler (20 mcg) |
||
| [n=486] | [n=491] | [n=483] | ||
| Patients with any adverse reaction | 46 | 52 | 45 | |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Cough Dyspnea | 3 2 | 2 2 | 2 3 |
|
| Nervous system disorders | ||||
| | Headache | 3 | 2 | 3 |
| Infections and infestations | ||||
| Bronchitis Nasopharyngitis Upper Respiratory infection | 3 4 3 | 3 3 4 | 1 4 3 |
|
Drug Interactions
8. Use In Specific Populations
Combivent Respimat Description
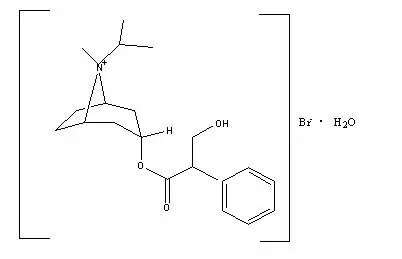
C20H30BrNO3•H2O ipratropium bromide Mol. Wt. 430.4
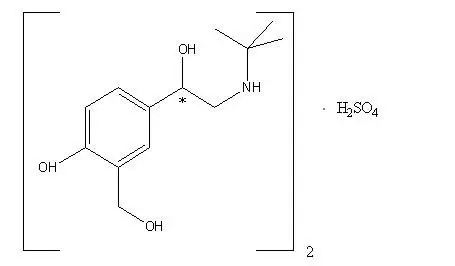
Combivent Respimat - Clinical Pharmacology
Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Adverse Effects Associated with Beta2-agonists
Hypersensitivity Reactions
Inform patients that hypersensitivity reactions, including urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema, may occur after the administration of COMBIVENT RESPIMAT. Advise patients to immediately discontinue COMBIVENT RESPIMAT and consult a physician [see Warnings and Precautions (5.6)].
Preparation for Use and Priming
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH
COMBIVENT® is a registered trademark of and used under license from Boehringer Ingelheim Pharmaceuticals, Inc.
Copyright © 2021 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
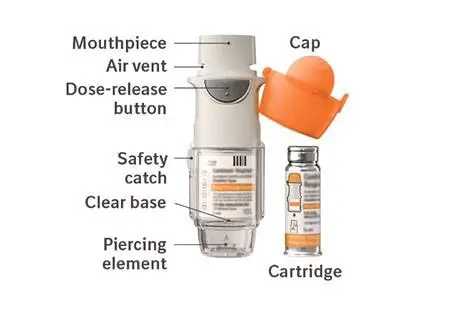
For Oral Inhalation Only
Do not spray COMBIVENT RESPIMAT into your eyes.
The COMBIVENT RESPIMAT inhaler has a slow moving mist that helps you inhale the medicine.
Do not turn the clear base before inserting the cartridge.
How to store your COMBIVENT RESPIMAT inhaler
- Store COMBIVENT RESPIMAT at room temperature 68°F to 77°F (20°C to 25°C).
- Do not freeze your COMBIVENT RESPIMAT cartridge and inhaler.
- If COMBIVENT RESPIMAT has not been used for more than 3 days, release 1 puff towards the ground.
- If COMBIVENT RESPIMAT has not been used for more than 21 days, repeat steps 4 to 6 under the “Prepare for first use” until a mist is visible. Then repeat steps 4 to 6 three more times.
- Keep your COMBIVENT RESPIMAT cartridge and inhaler out of the reach of children.
How to care for your COMBIVENT RESPIMAT inhaler
When to get a new COMBIVENT RESPIMAT inhaler
- Your inhaler contains 120 puffs (120 doses); or if you have a sample, your inhaler contains 60 puffs (60 doses) instead.
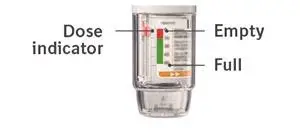
- The dose indicator shows approximately how much medicine is left.
- When the dose indicator enters the red area of the scale you need to get a refill; there is approximately medicine for 7 days left (if you have a sample, there is approximately medicine for 3 days left).
- When the dose indicator reaches the end of the red scale, your COMBIVENT RESPIMAT is empty and automatically locks. At this point, the clear base cannot be turned any further.
- Three months after insertion of cartridge, throw away the COMBIVENT RESPIMAT even if it has not been used, or when the inhaler is locked, or when it expires, whichever comes first.
Prepare for first use
1. Remove clear base
|  |
2. Insert cartridge
| 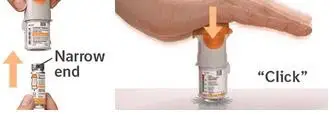 |
3. Replace clear base
|  |
4. Turn
|  |
5. Open
|  |
6. Press
|  |
Turn
| 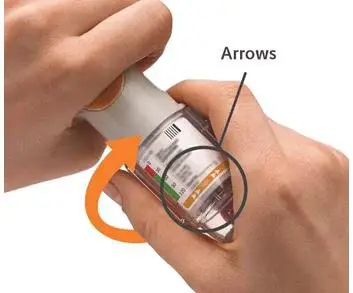 |
Open
|  |
Press
| 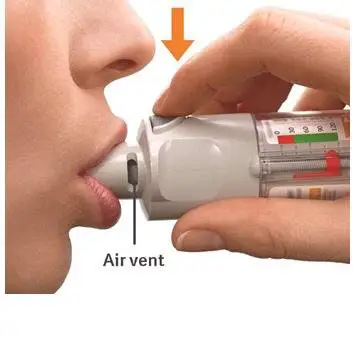 |
It is difficult to insert the cartridge deep enough:
I cannot press the dose-release button:
The dose indicator on the COMBIVENT RESPIMAT reaches zero too soon:
My COMBIVENT RESPIMAT sprays automatically:
Was the cap open when you turned the clear base? Close the cap, then turn the clear base.
My COMBIVENT RESPIMAT doesn’t spray:
Did you insert a cartridge? If not, insert a cartridge.

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
Licensed from: Boehringer Ingelheim International GmbH
| COMBIVENT RESPIMAT
ipratropium bromide and albuterol spray, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | API MANUFACTURE(0597-0024) , LABEL(0597-0024) , MANUFACTURE(0597-0024) , PACK(0597-0024) | |