Drug Detail:Consensi (Amlodipine and celecoxib)
Drug Class: Cox-2 inhibitors Miscellaneous antihypertensive combinations
Highlights of Prescribing Information
CONSENSI® (amlodipine and celecoxib) tablets, for oral administration. Initial U.S. Approval: 2018
WARNING: RISK OF SERIOUS CARDIOVASCULAR and GASTROINTESTINAL EVENTS
See full prescribing information for complete boxed warning.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in the treatment and may increase with duration of use. ( 5.1)
- CONSENSI is contraindicated in the setting of coronary artery bypass graft (CABG) surgery. ( 4, 5.1)
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events. ( 5.2)
Recent Major Changes
| Warnings and Precautions ( 5.1, 5.4) | 05/2020 |
| Warnings and Precautions ( 5.11, 5.12, 5.13) | 11/2020 |
Indications and Usage for Consensi
CONSENSI is a combination of amlodipine besylate, a calcium channel blocker, and celecoxib, a nonsteroidal anti-inflammatory drug (NSAID), indicated for patients for whom treatment with amlodipine for hypertension and celecoxib for osteoarthritis are appropriate. Lowering blood pressure reduces the risk of fatal and nonfatal CV events, primarily strokes and myocardial infarctions. ( 1.1)
Limitations of Use
CONSENSI is only available in a celecoxib strength of 200 mg and is only to be taken once daily. ( 1.1)
Consensi Dosage and Administration
Use the lowest effective dosage of celecoxib for the shortest duration consistent with individual treatment goals. If analgesic therapy is no longer indicated, discontinue CONSENSI and initiate patient on alternative antihypertensive therapy. ( 2.1, 2.2)
Start at (amlodipine/celecoxib) 5 mg/200 mg (2.5 mg/200 mg for small, elderly, or frail patients or hepatic impairment) orally once daily. Titrate to 5 mg/200 mg or 10 mg/200 mg once daily as needed for blood pressure control. ( 2.1)
CONSENSI may be substituted for its individual components. ( 2.3)
Dosage Forms and Strengths
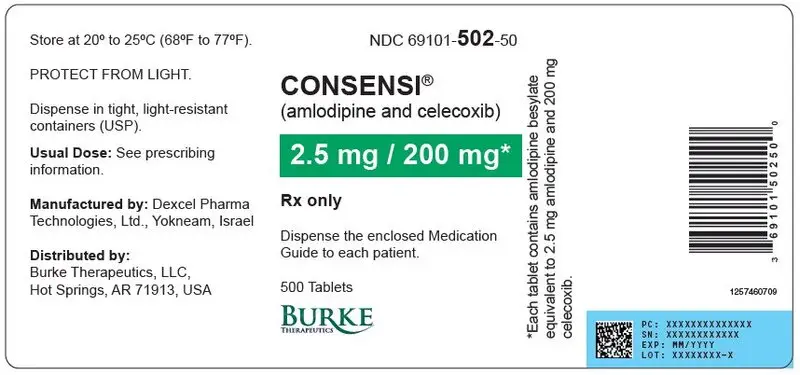
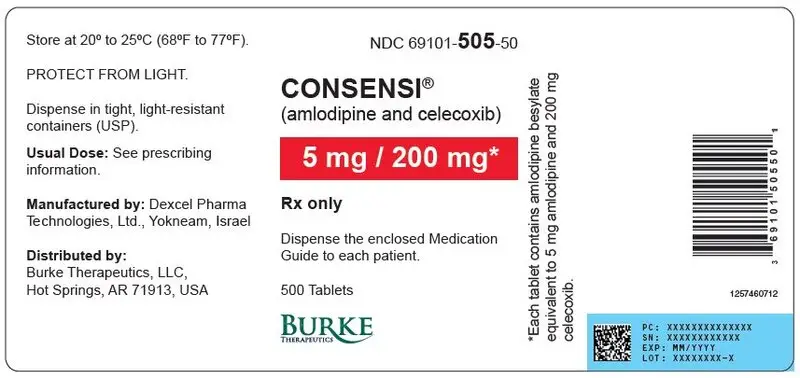
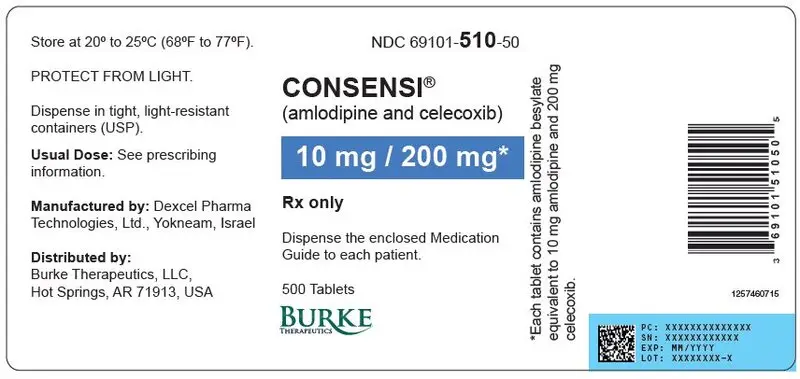
Tablets (amlodipine/celecoxib): 2.5 mg/200 mg, 5 mg/200 mg, or 10 mg/200 mg ( 3)
Contraindications
- Known hypersensitivity to amlodipine, celecoxib, or any inactive ingredients of CONSENSI ( 4)
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs ( 4)
- In the setting of CABG surgery ( 4)
- Demonstrated allergic-type reactions to sulfonamides ( 4)
Warnings and Precautions
- Hepatotoxicity and Patients with Hepatic Failure: Inform patients of warning signs and symptoms of hepatotoxicity. Discontinue if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop. ( 5.3)
- Hypertension: Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure. ( 5.4, 7)
- Hypotension: Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. ( 5.5)
- Increased Angina or Myocardial Infarction: Worsening angina and acute myocardial infarction, particularly in patients with severe obstructive coronary artery disease. ( 5.6)
- Heart Failure and Edema: Avoid use of CONSENSI in patients with severe heart failure. ( 5.7)
- Renal Toxicity: Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of CONSENSI in patients with advanced renal disease ( 5.8)
- Anaphylactic Reactions: Seek emergency help if an anaphylactic reaction occurs. ( 5.9)
- Exacerbation of Asthma Related to Aspirin Sensitivity: CONSENSI is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin sensitivity). ( 5.10)
- Serious Skin Reactions: Discontinue CONSENSI at first appearance of skin rash or other signs of hypersensitivity. ( 5.11)
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Discontinue and evaluate clinically ( 5.12).
- Fetal Toxicity: Limit use of NSAIDs, including CONSENSI, between about 20 to 30 weeks in pregnancy due to the risk of oligohydramnios/fetal renal dysfunction. Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy due to the risks of oligohydramnios/fetal renal dysfunction and premature closure of the fetal ductus arteriosus ( 5.13, 8.1)
- Hematologic Toxicity: Monitor hemoglobin or hematocrit in patients with any signs or symptoms of anemia. ( 5.14, 7)
Adverse Reactions/Side Effects
Most common adverse reactions to celecoxib in arthritis trials (>2% and >placebo): abdominal pain, diarrhea, dyspepsia, flatulence, peripheral edema, accidental injury, dizziness, pharyngitis, rhinitis, sinusitis, upper respiratory tract infection, rash. ( 6.1)
Most common adverse reaction to amlodipine is edema which occurred in a dose related manner. Other adverse experiences to amlodipine that were not dose related but that were reported with an incidence >1.0% are fatigue, nausea, abdominal pain, and somnolence. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Burke Therapeutics, LLC at 1-888-275-1264 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Drugs that Interfere with Hemostasis (e.g. warfarin, aspirin, SSRIs/SNRIs): Monitor patients for bleeding who are concomitantly taking CONSENSI with drugs that interfere with hemostasis. Concomitant use of CONSENSI and analgesic doses of aspirin is not generally recommended. ( 7)
- ACE Inhibitors, Angiotensin Receptor Blockers (ARB), or Beta-Blockers: Concomitant use with CONSENSI may diminish the antihypertensive effect of these drugs. Monitor blood pressure. ( 7)
- ACE Inhibitors and ARBs: Concomitant use with CONSENSI in elderly, volume depleted, or those with renal impairment may result in deterioration of renal function. In such high risk patients, monitor for signs of worsening renal function. ( 7)
- Diuretics: NSAIDs can reduce natriuretic effect of furosemide and thiazide diuretics. Monitor patients to assure diuretic efficacy including antihypertensive effects. ( 7)
- Digoxin: Concomitant use with CONSENSI can increase serum concentration and prolong half-life of digoxin. Monitor serum digoxin levels. ( 7)
- Simvastatin: Do not exceed 20 mg of simvastatin per day in patients taking amlodipine. ( 7)
Use In Specific Populations
- Infertility: NSAIDs are associated with reversible infertility. ( 8.3)
- Hepatic or Renal Impairment: Not recommended in patients with moderate or severe hepatic impairment or severe renal insufficiency.
- Poor Metabolizers of CYP2C9 Substrates: Not recommended.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2021
Full Prescribing Information
1. Indications and Usage for Consensi
2. Consensi Dosage and Administration
2.1 Recommended Dosage
Use the lowest effective dosage of celecoxib for the shortest duration consistent with individual patient treatment goals [see Dosage and Administration (2.2) and Warnings and Precautions (5)] . Only 200 mg of celecoxib once daily is available with CONSENSI.
Start CONSENSI in adults at (amlodipine/celecoxib) 5 mg/200 mg orally once daily or 2.5 mg/200 mg in small, fragile, or elderly patients, or patients with mild hepatic insufficiency. Use 2.5 mg/200 mg when adding CONSENSI to other antihypertensive therapy.
Adjust amlodipine component dosage according to blood pressure goals. In general, wait 7 to 14 days between titration steps. If more rapid titration is clinically warranted, monitor closely. The maximum dose is 10 mg/200 mg once daily.
3. Dosage Forms and Strengths
CONSENSI (amlodipine and celecoxib) tablets are white and biconvex, non-coated, non-scored, with the tablet strength debossed on one side, available in the following strengths:
| Amlodipine/Celecoxib | Shape |
|---|---|
| 2.5 mg/200 mg | Elongated oval |
| 5 mg/200 mg | Caplet |
| 10 mg/200 mg | Round |
4. Contraindications
CONSENSI is contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to amlodipine, celecoxib, or any of the inactive ingredients in CONSENSI [see Warnings and Precautions (5.9, 5.11)] .
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs, have been reported in such patients [see Warnings and Precautions (5.9, 5.10)] .
- In the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.1)] .
- In patients who have demonstrated allergic-type reactions to sulfonamides [see Warnings and Precautions (5.9)] .
5. Warnings and Precautions
5.1 Cardiovascular Thrombotic Events
5.2 Gastrointestinal Bleeding, Ulceration, and Perforation
5.8 Renal Toxicity and Hyperkalemia
Celecoxib
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events [see Warnings and Precautions (5.1)]
- GI Bleeding, Ulceration and Perforation [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.3)]
- Hypertension [see Warnings and Precautions (5.4)]
- Hypotension [see Warnings and Precautions (5.5)]
- Increased Angina or Myocardial Infarction [see Warnings and Precautions (5.6)]
- Heart Failure and Edema [see Warnings and Precautions (5.7)]
- Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.8)]
- Anaphylactic Reactions [see Warnings and Precautions (5.9)]
- Serious Skin Reactions [see Warnings and Precautions (5.11)]
- Hematologic Toxicity [see Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Celecoxib Clinical Trials
Of the celecoxib-treated patients in the pre-marketing controlled clinical trials, approximately 4,250 were patients with osteoarthritis, approximately 2,100 were patients with rheumatoid arthritis, and approximately 1,050 were patients with post-surgical pain. More than 8,500 patients received a total daily dose of celecoxib of 200 mg (100 mg twice daily or 200 mg once daily) or more, including more than 400 treated at 800 mg (400 mg twice daily). Approximately 3,900 patients received celecoxib at these doses for 6 months or more; approximately 2,300 of these have received it for 1 year or more and 124 of these have received it for 2 years or more.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of either celecoxib or amlodipine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7. Drug Interactions
8. Use In Specific Populations
8.1 Pregnancy
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including CONSENSI, can cause premature closure of the fetal ductus arteriosus
(see
Data)
.
Oligohydramnios/Neonatal Renal Impairment
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If CONSENSI treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue CONSENSI and follow up according to clinical practice
(see
Data)
.
8.8 Poor Metabolizers of CYP2C9 Substrates
In patients who are known or suspected to be poor CYP2C9 metabolizers (i.e., CYP2C9*3/*3), based on genotype or previous history/experience with other CYP2C9 substrates (such as warfarin, phenytoin) administer celecoxib starting with half the lowest recommended dose. Because CONSENSI is not available in lower strengths of celecoxib, CONSENSI is not recommended in patients who are known or suspected to be poor CYP2C9 metabolizers [see Clinical Pharmacology (12.5)] .
11. Consensi Description
CONSENSI (amlodipine and celecoxib) tablet is an NSAID and long-acting calcium channel blocker for oral administration. Each tablet contains amlodipine besylate and celecoxib 3.47 mg/200 mg, 6.93 mg/200 mg, and 13.87 mg/200 mg and is equivalent to 2.5 mg/200 mg, 5 mg/200 mg, and 10 mg/200 mg amlodipine/celecoxib respectively.
Celecoxib is chemically designated as 4-[5-(4-methylphenyl)- 3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide and is a diaryl-substituted pyrazole. The empirical formula is C 17H 14F 3N 3O 2S, and the molecular weight is 381.38; the chemical structure is as follows:
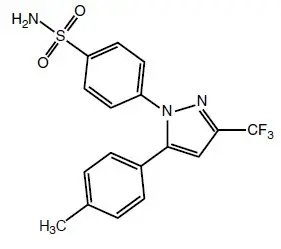
Celecoxib is a white to off-white powder with a pKa of 11.1 (sulfonamide moiety). Celecoxib is hydrophobic (log P is 3.5) and is practically insoluble in aqueous media at physiological pH range.
Amlodipine besylate is chemically designated as 3-Ethyl-5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. The molecular formula is C 20H 25ClN 2O 5∙C 6H 6O 3S, and the molecular weight is 567.1; the chemical structure is as follows:
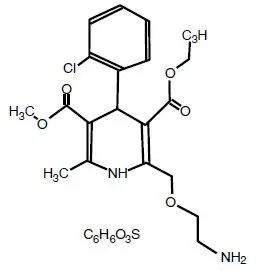
Amlodipine besylate is a white crystalline powder. It is slightly soluble in water and sparingly soluble in ethanol.
The inactive ingredients in CONSENSI include: mannitol DC 200, croscarmellose sodium, povidone K-30, sodium lauryl sulfate, magnesium stearate, and colloidal silicon dioxide.
12. Consensi - Clinical Pharmacology
12.3 Pharmacokinetics
Celecoxib
Celecoxib exhibits dose-proportional increase in exposure after oral administration up to 200 mg twice daily and less than proportional increase at higher doses. It has extensive distribution and high protein binding. It is primarily metabolized by CYP2C9 with a half-life of approximately 11 hours.
Amlodipine
After oral administration of therapeutic doses of amlodipine, absorption produces peak plasma concentrations between 6 and 12 hours. Absolute bioavailability has been estimated to be between 64 and 90%.
Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. Ex vivo studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Elimination from the plasma is biphasic with a terminal elimination half-life of about 30– 50 hours. Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing.
The pharmacokinetics of amlodipine are not significantly influenced by renal impairment. Patients with renal failure may therefore receive the usual initial dose.
Elderly patients and patients with hepatic insufficiency have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40– 60%, and a lower initial dose may be required. A similar increase in AUC was observed in patients with moderate to severe heart failure.
Sixty-two hypertensive patients aged 6 to 17 years received doses of amlodipine between 1.25 mg and 20 mg. Weight-adjusted clearance and volume of distribution were similar to values in adults.
Drug Interactions
In vitro data indicate that amlodipine has no effect on the human plasma protein binding of digoxin, phenytoin, warfarin, and indomethacin.
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Combination of Celecoxib and Amlodipine
During the development of this fixed-dose combination product, the central focus was to assess pharmacodynamic interactions related to blood pressure effect between celecoxib and amlodipine. There are no studies of the combination of celecoxib and amlodipine demonstrating reductions in the signs and symptoms of osteoarthritis, but one of the components, celecoxib, has demonstrated such effects. There are also no long-term studies to evaluate CV safety for the combination of celecoxib and amlodipine.
The combination of celecoxib and amlodipine was studied in a randomized, double-blind, placebo- and active-controlled study in 152 patients with newly diagnosed hypertension who required pharmacological therapy to control their hypertension. A total of 63% of patients were male, 25% were 65 years or older, 95% were white, 2% were black, and 3% were Asian. The trial used commercial celecoxib capsules and amlodipine tablets that were individually over encapsulated and then taken together or with matching placebos. The patients were randomized 1.5:1.5:1:1 to one of four treatment arms: 200 mg celecoxib + 10 mg amlodipine (celecoxib + amlodipine arm), 0 mg celecoxib + 10 mg amlodipine (amlodipine arm), 200 mg celecoxib + 0 mg amlodipine (celecoxib arm), and 0 mg celecoxib and 0 mg amlodipine (placebo arm). All drugs were administered once a day for 14 days. The trial results demonstrated that the combination of celecoxib and amlodipine provided similar blood pressure reduction to an equal dose of amlodipine.
14.3 Special Studies
Celecoxib
Cardiovascular Outcomes Trial: Prospective Randomized Evaluation of Celecoxib Integrated Safety vs. Ibuprofen Or Naproxen(PRECISION; NCT00346216)
Results
Among subjects with osteoarthritis, only 0.2% (17/7259) escalated celecoxib to the 200 mg twice daily dose, whereas 54.7% (3946/7208) escalated ibuprofen to 800 mg three times daily, and 54.8% (3937/7178) escalated naproxen to the 500 mg twice daily dose. Among subjects with rheumatoid arthritis, 55.7% (453/813) escalated celecoxib to the 200 mg twice daily dose, 56.5% (470/832) escalated ibuprofen to 800 mg three times daily, and 54.6% (432/791) escalated naproxen to the 500 mg twice daily dose; however, the rheumatoid arthritis population accounted for only 10% of the trial population.
Because relatively few celecoxib patients overall (5.8% [470/8072]) dose-escalated to 200 mg twice daily, the results of the PRECISION trial are not suitable for determining the relative CV safety of celecoxib at 200 mg twice daily compared to ibuprofen and naproxen at the doses taken.
16. How is Consensi supplied
CONSENSI tablets are white and biconvex, non-coated, non-scored, with the tablet strength debossed on one side, available as follows:
| Amlodipine | Celecoxib | Shape | NDC | |
|---|---|---|---|---|
| Bottle of 30 tablets | Bottle of 500 tablets | |||
| 2.5 mg | 200 mg | Elongated oval | 69101- 502-30 | 69101- 502-50 |
| 5 mg | 200 mg | Caplet | 69101- 505-30 | 69101- 505-50 |
| 10 mg | 200 mg | Round | 69101- 510-30 | 69101- 510-50 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with CONSENSI and periodically during therapy.
| CONSENSI
amlodipine besylate and celecoxib tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CONSENSI
amlodipine besylate and celecoxib tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CONSENSI
amlodipine besylate and celecoxib tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Burke Therapeutics, LLC (079259903) |