Drug Detail:Copiktra (Duvelisib [ doo-ve-lis-ib ])
Drug Class: PI3K inhibitors
Highlights of Prescribing Information
COPIKTRA (duvelisib), capsules for oral use
Initial U.S. Approval: 2018
WARNING: FATAL AND SERIOUS TOXICITIES: INFECTIONS, DIARRHEA OR COLITIS, CUTANEOUS REACTIONS, and PNEUMONITIS
See full prescribing information for complete boxed warning
- Fatal and/or serious infections occurred in 31% of COPIKTRA-treated patients. Monitor for signs and symptoms of infection. Withhold COPIKTRA if infection is suspected. (5.1)
- Fatal and/or serious diarrhea or colitis occurred in 18% of COPIKTRA-treated patients. Monitor for the development of severe diarrhea or colitis. Withhold COPIKTRA. (5.2)
- Fatal and/or serious cutaneous reactions occurred in 5% of COPIKTRA-treated patients. Withhold COPIKTRA. (5.3)
- Fatal and/or serious pneumonitis occurred in 5% of COPIKTRA-treated patients. Monitor for pulmonary symptoms and interstitial infiltrates. Withhold COPIKTRA. (5.4)
Recent Major Changes
Indications and Usage, Follicular Lymphoma (1.2) Removed 12/2021
Dosage and Administration, Dose Modifications for Concomitant Use with CYP3A4 Inducers (2.5) 9/2021
Indications and Usage for Copiktra
COPIKTRA is a kinase inhibitor indicated for the treatment of adult patients with:
- relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) after at least two prior therapies. (1)
Copiktra Dosage and Administration
25 mg orally, twice daily. Modify dosage for toxicity. (2.1, 2.2)
Dosage Forms and Strengths
Capsules: 25 mg, 15 mg. (3)
Contraindications
None. (4)
Warnings and Precautions
- Hepatotoxicity: Monitor hepatic function. (5.5)
- Neutropenia: Monitor blood counts. (5.6)
- Embryo-Fetal toxicity: COPIKTRA can cause fetal harm. Advise females of reproductive potential and males with female partners of reproductive potential of potential risk to a fetus and to use effective contraception. (5.7)
Adverse Reactions/Side Effects
The most common adverse reactions (> 20%) are diarrhea or colitis, neutropenia, rash, fatigue, pyrexia, cough, nausea, upper respiratory infection, pneumonia, musculoskeletal pain, and anemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Secura Bio, Inc. (Secura Bio) at 1-844-973-2872, or U.S. Food and Drug Administration (FDA) at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 inhibitors: Reduce COPIKTRA dose to 15 mg twice daily when co-administered with strong CYP3A4 inhibitors. (2.4, 7.1, 12.3)
- Strong CYP3A4 inducers: Avoid coadministration. (2.5, 7.1, 12.3)
- Moderate CYP3A4 inducers: Avoid coadministration. If coadministration cannot be avoided, increase the dose of COPIKTRA. (2.5, 7.1, 12.3)
- CYP3A4 substrates: Monitor for signs of toxicities when co-administering COPIKTRA with sensitive CYP3A substrates. (7.2)
Use In Specific Populations
Lactation: Advise women not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2021
Full Prescribing Information
WARNING: FATAL AND SERIOUS TOXICITIES: INFECTIONS, DIARRHEA OR COLITIS, CUTANEOUS REACTIONS, and PNEUMONITIS
- Fatal and/or serious infections occurred in 31% of COPIKTRA-treated patients. Monitor for signs and symptoms of infection. Withhold COPIKTRA if infection is suspected [see Warnings and Precautions (5.1)].
- Fatal and/or serious diarrhea or colitis occurred in 18% of COPIKTRA-treated patients. Monitor for the development of severe diarrhea or colitis. Withhold COPIKTRA [see Warnings and Precautions (5.2)] .
- Fatal and/or serious cutaneous reactions occurred in 5% of COPIKTRA-treated patients. Withhold COPIKTRA [see Warnings and Precautions (5.3)].
- Fatal and/or serious pneumonitis occurred in 5% of COPIKTRA-treated patients. Monitor for pulmonary symptoms and interstitial infiltrates. Withhold COPIKTRA [see Warnings and Precautions (5.4)].
2. Copiktra Dosage and Administration
2.1 Dosing
Advise patients that if a dose is missed by fewer than 6 hours, to take the missed dose right away and take the next dose as usual. If a dose is missed by more than 6 hours, advise patients to wait and take the next dose at the usual time.
2.2 Recommended Prophylaxis
Withhold COPIKTRA in patients with suspected PJP of any grade, and discontinue if PJP is confirmed.
Consider prophylactic antivirals during COPIKTRA treatment to prevent cytomegalovirus (CMV) infection including CMV reactivation.
2.3 Dose Modifications for Adverse Reactions
Manage toxicities per Table 1 with dose reduction, treatment hold, or discontinuation of COPIKTRA.
|
Abbreviations: ALT = alanine aminotransferase; ANC = absolute neutrophil count; AST = aspartate aminotransferase; |
||
|
CMV = cytomegalovirus; DRESS = drug reaction with eosinophilia and systemic systems; PCR = polymerase chain reaction; PJP = Pneumocystis jirovecii; pneumonia; SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis; ULN = upper limit of normal |
||
| Toxicity
| Adverse Reaction Grade
| Recommended Management
|
| Nonhematologic Adverse Reactions
|
||
| Infections | Grade 3 or higher infection |
|
| Clinical CMV infection or viremia (positive PCR or antigen test) |
|
|
| PJP |
|
|
| Non-infectious Diarrhea or colitis | Mild/moderate diarrhea (Grade 1-2, up to 6 stools per day over baseline) and responsive to antidiarrheal agents, OR Asymptomatic (Grade 1) colitis |
|
| Mild/moderate diarrhea (Grade 1-2, up to 6 stools per day over baseline) and unresponsive to antidiarrheal agents |
|
|
| Abdominal pain, stool with mucus or blood, change in bowel habits, peritoneal signs, OR Severe diarrhea (Grade 3, >6 stools per day over baseline) |
|
|
| Life-threatening |
|
|
| Cutaneous reactions | Grade 1-2 |
|
| Grade 3 |
|
|
| Life-threatening |
|
|
| SJS, TEN, DRESS (any grade) |
|
|
| Pneumonitis without suspected infectious cause | Moderate (Grade 2) symptomatic pneumonitis |
|
| Severe (Grade 3) or life-threatening pneumonitis |
|
|
| ALT/AST elevation | 3 to 5 × upper limit of normal (ULN) (Grade 2) |
|
| > 5 to 20 × ULN (Grade 3) |
|
|
| > 20 × ULN (Grade 4) |
|
|
| Hematologic Adverse Reactions
|
||
| Neutropenia | Absolute neutrophil count (ANC) 0.5 to 1.0 Gi/L |
|
| ANC less than 0.5 Gi/L |
|
|
| Thrombocytopenia | Platelet count 25 to < 50 Gi/L (Grade 3) with Grade 1 bleeding |
|
| Platelet count 25 to < 50 Gi/L (Grade 3) with Grade 2 bleeding or Platelet count < 25 Gi/L (Grade 4) |
|
|
Recommended dose modification levels for COPIKTRA are presented in Table 2.
| Dose Level
| Dose
|
| Initial Dose | 25 mg twice daily |
| Dose Reduction | 15 mg twice daily |
| Subsequent Dose Modification | Discontinue COPIKTRA if patient is unable to tolerate 15 mg twice daily. |
2.5 Dosage Modification for Concomitant Use with CYP3A4 Inducers
Avoid coadministration of COPIKTRA with strong CYP3A4 inducers.
Avoid coadministration of COPIKTRA with moderate CYP3A4 inducers. If coadministration with a moderate CYP3A4 inducer cannot be avoided, increase the COPIKTRA dose on Day 12 of coadministration with the moderate CYP3A4 inducer as recommended in Table 3.
| Initial COPIKTRA Dosage | Recommended COPIKTRA Dosage |
| 25 mg orally twice daily | 40 mg orally twice daily |
| 15 mg orally twice daily | 25 mg orally twice daily |
3. Dosage Forms and Strengths
| Strength
| Description
|
| 25 mg | White to off-white opaque and Swedish orange opaque capsule printed in black ink with "duv 25 mg" |
| 15 mg | Pink opaque capsule printed in black ink with "duv 15 mg" |
5. Warnings and Precautions
5.1 Infections
Treat infections prior to initiation of COPIKTRA. Advise patients to report any new or worsening signs and symptoms of infection. For grade 3 or higher infection, withhold COPIKTRA until infection has resolved. Resume COPIKTRA at the same or reduced dose [see Dosage and Administration (2.3)].
Serious, including fatal, Pneumocystis jirovecii pneumonia (PJP) occurred in 1% of patients taking COPIKTRA. Provide prophylaxis for PJP during treatment with COPIKTRA. Following completion of COPIKTRA treatment, continue PJP prophylaxis until the absolute CD4+ T cell count is greater than 200 cells/µL. Withhold COPIKTRA in patients with suspected PJP of any grade, and permanently discontinue if PJP is confirmed.
CMV reactivation/infection occurred in 1% of patients taking COPIKTRA. Consider prophylactic antivirals during COPIKTRA treatment to prevent CMV infection including CMV reactivation. For clinical CMV infection or viremia, withhold COPIKTRA until infection or viremia resolves. If COPIKTRA is resumed, administer the same or reduced dose and monitor patients for CMV reactivation by PCR or antigen test at least monthly [see Dosage and Administration (2.3)].
5.2 Diarrhea or Colitis
Advise patients to report any new or worsening diarrhea. For non-infectious diarrhea or colitis, follow the guidelines below:
For patients presenting with mild or moderate diarrhea (Grade 1-2) (i.e. up to 6 stools per day over baseline) or asymptomatic (Grade 1) colitis, initiate supportive care with antidiarrheal agents as appropriate, continue COPIKTRA at the current dose, and monitor the patient at least weekly until the event resolves. If the diarrhea is unresponsive to antidiarrheal therapy, withhold COPIKTRA and initiate supportive therapy with enteric acting steroids (e.g. budesonide). Monitor the patient at least weekly. Upon resolution of the diarrhea, consider restarting COPIKTRA at a reduced dose.
For patients presenting with abdominal pain, stool with mucus or blood, change in bowel habits, peritoneal signs, or with severe diarrhea (Grade 3) (i.e. > 6 stools per day over baseline) withhold COPIKTRA and initiate supportive therapy with enteric acting steroids (e.g. budesonide) or systemic steroids. A diagnostic work-up to determine etiology, including colonoscopy, should be performed. Monitor at least weekly. Upon resolution of the diarrhea or colitis, restart COPIKTRA at a reduced dose. For recurrent Grade 3 diarrhea or recurrent colitis of any grade, discontinue COPIKTRA. Discontinue COPIKTRA for life-threatening diarrhea or colitis [see Dosage and Administration (2.3)].
5.3 Cutaneous Reactions
Presenting features for the serious events were primarily described as pruritic, erythematous, or maculo-papular. Less common presenting features include exanthem, desquamation, erythroderma, skin exfoliation, keratinocyte necrosis, and papular rash. Advise patients to report any new or worsening cutaneous reactions. Review all concomitant medications and discontinue any medications potentially contributing to the event. For patients presenting with mild or moderate (Grade 1-2) cutaneous reactions, continue COPIKTRA at the current dose, initiate supportive care with emollients, anti-histamines (for pruritus), or topical steroids, and monitor the patient closely. Withhold COPIKTRA for severe (Grade 3) cutaneous reaction until resolution. Initiate supportive care with steroids (topical or systemic) or anti-histamines (for pruritus). Monitor at least weekly until resolved. Upon resolution of the event, restart COPIKTRA at a reduced dose. Discontinue COPIKTRA if severe cutaneous reaction does not improve, worsens, or recurs. For life-threatening cutaneous reactions, discontinue COPIKTRA. In patients with SJS, TEN, or DRESS of any grade, discontinue COPIKTRA [see Dosage and Administration (2.3)].
5.4 Pneumonitis
Withhold COPIKTRA in patients who present with new or progressive pulmonary signs and symptoms such as cough, dyspnea, hypoxia, interstitial infiltrates on a radiologic exam, or a decline by more than 5% in oxygen saturation and evaluate for etiology. If the pneumonitis is infectious, patients may be restarted on COPIKTRA at the previous dose once the infection, pulmonary signs and symptoms resolve. For moderate non-infectious pneumonitis (Grade 2), treat with systemic corticosteroids, and resume COPIKTRA at a reduced dose upon resolution. If non-infectious pneumonitis recurs or does not respond to steroid therapy, discontinue COPIKTRA. For severe or life-threatening non-infectious pneumonitis, discontinue COPIKTRA and treat with systemic steroids [see Dosage and Administration (2.3)].
5.5 Hepatotoxicity
Monitor hepatic function during treatment with COPIKTRA. For Grade 2 ALT/AST elevation (greater than 3 to 5 × ULN), maintain COPIKTRA dose and monitor at least weekly until return to less than 3 × ULN. For Grade 3 ALT/AST elevation (greater than 5 to 20 × ULN), withhold COPIKTRA and monitor at least weekly until return to less than 3 × ULN. Resume COPIKTRA at the same dose (first occurrence) or at a reduced dose for subsequent occurrence. For grade 4 ALT/AST elevation (greater than 20 × ULN) discontinue COPIKTRA [see Dosage and Administration (2.3)].
5.6 Neutropenia
Monitor neutrophil counts at least every 2 weeks for the first 2 months of COPIKTRA therapy, and at least weekly in patients with neutrophil counts < 1.0 Gi/L (Grade 3-4). Withhold COPIKTRA in patients presenting with neutrophil counts < 0.5 Gi/L (Grade 4). Monitor until ANC is > 0.5 Gi/L, resume COPIKTRA at same dose for the first occurrence or a reduced dose for subsequent occurrence [see Dosage and Administration (2.3)].
6. Adverse Reactions/Side Effects
- Infections [see Warnings and Precautions (5.1)]
- Diarrhea or Colitis [see Warnings and Precautions (5.2)]
- Cutaneous Reactions [see Warnings and Precautions (5.3)]
- Pneumonitis [see Warnings and Precautions (5.4)]
- Hepatotoxicity [see Warnings and Precautions (5.5)]
- Neutropenia [see Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Summary of Clinical Trial Experience in B-cell Malignancies
The data described below reflect exposure to COPIKTRA in two single-arm, open-label clinical trials, one open-label extension clinical trial, and one randomized, open-label, actively controlled clinical trial totaling 442 patients with previously treated hematologic malignancies primarily including CLL/SLL (69%) and FL (22%). Patients were treated with COPIKTRA 25 mg BID until unacceptable toxicity or progressive disease. The median duration of exposure was 9 months (range: 0.1 to 53 months), with 36% (160/442) of patients having at least 12 months of exposure.
For the 442 patients, the median age was 67 years (range: 30 to 90 years), 65% were male, 92% were White, and 93% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. Patients had a median of 2 prior therapies. The trials required hepatic transaminases at least ≤ 3 times upper limit of normal (ULN), total bilirubin ≤ 1.5 times ULN, and serum creatinine ≤ 1.5 times ULN. Patients were excluded for prior exposure to a PI3K inhibitor within 4 weeks.
Fatal adverse reactions within 30 days of the last dose occurred in 36 patients (8%) treated with COPIKTRA 25 mg BID.
Serious adverse reactions were reported in 289 patients (65%). The most frequent serious adverse reactions that occurred were infection (31%), diarrhea or colitis (18%), pneumonia (17%), rash (5%), and pneumonitis (5%).
Adverse reactions resulted in treatment discontinuation in 156 patients (35%), most often due to diarrhea or colitis, infection, and rash. COPIKTRA was dose reduced in 104 patients (24%) due to adverse reactions, most often due to diarrhea or colitis and transaminase elevation. The median time to first dose modification or discontinuation was 4 months (range: 0.1 to 27 months), with 75% of patients having their first dose modification or discontinuation within 7 months.
Common Adverse Reactions
Table 4 summarizes common adverse reactions in patients receiving COPIKTRA 25 mg BID, and Table 5 summarizes the treatment-emergent laboratory abnormalities. The most common adverse reactions (reported in ≥ 20% of patients) were diarrhea or colitis, neutropenia, rash, fatigue, pyrexia, cough, nausea, upper respiratory infection, pneumonia, musculoskeletal pain, and anemia.
|
a Diarrhea or colitis includes the preferred terms: colitis, enterocolitis, colitis microscopic, colitis ulcerative, diarrhea, diarrhea hemorrhagic |
||
|
b Transaminase elevation includes the preferred terms: alanine aminotransferase increased, aspartate aminotransferase increased, transaminases increased, hypertransaminasemia, hepatocellular injury, hepatotoxicity |
||
|
c Pneumonia includes the preferred terms: All preferred terms containing "pneumonia" except for "pneumonia aspiration"; bronchopneumonia, bronchopulmonary aspergillosis |
||
|
d Rash includes the preferred terms: dermatitis (including allergic, exfoliative, perivascular), erythema (including multiforme), rash (including exfoliative, erythematous, follicular, generalized, macular & papular, pruritic, pustular), toxic epidermal necrolysis and toxic skin eruption, drug reaction with eosinophilia and systemic symptoms, drug eruption, Stevens-Johnson syndrome |
||
| Adverse Reactions
| COPIKTRA 25 mg BID
(N = 442) |
|
| Any Grade
n (%) | Grade ≥ 3
n (%) |
|
| Blood and lymphatic system disorders
| ||
| Neutropenia † Anemia † Thrombocytopenia † | 151 (34) 90 (20) 74 (17) | 132 (30) 48 (11) 46 (10) |
| Gastrointestinal disorders
| ||
| Diarrhea or colitis †a
Nausea † Abdominal pain Vomiting Mucositis Constipation | 222 (50) 104 (24) 78 (18) 69 (16) 61 (14) 57 (13) | 101 (23) 4 (< 1) 9 (2) 6 (1) 6 (1) 1 (< 1) |
| General disorders and administration site conditions
| ||
| Fatigue † Pyrexia | 126 (29) 115 (26) | 22 (5) 7 (2) |
| Hepatobiliary disorders
| ||
| Transaminase elevation †b
| 67 (15) | 34 (8) |
| Infections and infestations
| ||
| Upper respiratory tract infection † Pneumonia †c Lower respiratory tract infection † | 94 (21) 91 (21) 46 (10) | 2 (< 1) 67 (15) 11 (3) |
| Metabolism and nutrition disorders
| ||
| Decreased appetite Edema † Hypokalemia † | 63 (14) 60 (14) 45 (10) | 2 (< 1) 6 (1) 17 (4) |
| Musculoskeletal and connective tissue disorders
| ||
| Musculoskeletal pain † Arthralgia | 90 (20) 46 (10) | 6 (1) 1 (< 1) |
| Nervous system disorders
| ||
| Headache † | 55 (12) | 1 (< 1) |
| Respiratory, thoracic and mediastinal disorders
| ||
| Cough † Dyspnea † | 111 (25) 52 (12) | 2 (< 1) 8 (2) |
| Skin and subcutaneous tissue disorders
| ||
| Rash †d
| 136 (31) | 41 (9) |
|
a Includes laboratory abnormalities that are new or worsening in grade or with worsening from baseline unknown. |
||
|
b Percentages are based on number of patients with at least one post-baseline assessment; not all patients were evaluable. |
||
| Laboratory Parameter a
| COPIKTRA 25 mg BID
(N = 442) |
|
| Any Grade
n (%)b | Grade ≥ 3
n (%)b |
|
| Hematology abnormalities
| ||
| Neutropenia Anemia Thrombocytopenia Lymphocytosis Leukopenia Lymphopenia | 276 (63) 198 (45) 170 (39) 132 (30) 129 (29) 90 (21) | 184 (42) 66 (15) 65 (15) 92 (21) 34 (8) 39 (9) |
| Chemistry abnormalities
| ||
| ALT increased AST increased Lipase increased Hypophosphatemia ALP increased Serum amylase increased Hyponatremia Hyperkalemia Hypoalbuminemia Creatinine increased Hypocalcemia | 177 (40) 163 (37) 133 (36) 136 (31) 128 (29) 101 (28) 116 (27) 114 (26) 111 (25) 106 (24) 100 (23) | 34 (8) 24 (6) 58 (16) 23 (5) 7 (2) 16 (4) 30 (7) 14 (3) 7 (2) 7 (2) 12 (3) |
Summary of Clinical Trial Experience in CLL/SLL
Study 1
The safety data below reflects exposure in a randomized, open-label, actively controlled clinical trial for adult patients with CLL or SLL who received at least one prior therapy. Of 313 patients treated, 158 received COPIKTRA monotherapy and 155 received ofatumumab. The 442-patient safety analysis above includes patients from Study 1.
COPIKTRA was administered at 25 mg BID in 28-day treatment cycles until unacceptable toxicity or progressive disease. The comparator group received 12 doses of ofatumumab with an initial dose of 300 mg intravenous (IV) on Day 1 followed a week later by 7 weekly doses of 2000 mg IV, followed 4 weeks later by 2000 mg IV every 4 weeks for 4 doses.
In the total study population, the median age was 69 years (range: 39 to 90 years), 60% were male, 92% were White, and 91% had an ECOG performance status of 0 to 1. Patients had a median of 2 prior therapies, with 61% of patients having received 2 or more prior therapies. The trial required a hemoglobin ≥ 8 g/dL and platelets ≥ 10,000 µL with or without transfusion support, hepatic transaminases ≤ 3 times upper limit of normal (ULN), total bilirubin ≤ 1.5 times ULN, and serum creatinine ≤ 2 times ULN. The trial excluded patients with prior autologous transplant within 6 months or allogeneic transplant, prior exposure to a PI3K inhibitor or a Bruton's tyrosine kinase (BTK) inhibitor, and uncontrolled autoimmune hemolytic anemia or idiopathic thrombocytopenic purpura.[see Clinical Studies (14)]
During randomized treatment, the median duration of exposure to COPIKTRA was 11.6 months with 72% (114/158) exposed for ≥ 6 months and 49% (77/158) exposed for ≥ 1 year. The median duration of exposure to ofatumumab was 5.3 months, with 77% (120/155) receiving at least 10 of 12 doses.
Fatal adverse reactions within 30 days of the last dose occurred in 12% (19/158) of patients treated with COPIKTRA and in 4% (7/155) of patients treated with ofatumumab.
Serious adverse reactions were reported in 73% (115/158) of patients treated with COPIKTRA and most often involved infection (38% of patients; 60/158) and diarrhea or colitis (23% of patients; 36/158).
COPIKTRA was discontinued in 57 patients (36%), most often due to diarrhea or colitis, infection, and rash. COPIKTRA was dose reduced in 46 patients (29%) due to adverse reactions, most often due to diarrhea or colitis and rash.
Common Adverse Reactions
Table 6 summarizes selected adverse reactions in Study 1, and Table 7 summarizes treatment-emergent laboratory abnormalities. The most common adverse reactions with COPIKTRA (reported in ≥ 20% of patients) were diarrhea or colitis, neutropenia, pyrexia, upper respiratory tract infection, pneumonia, rash, fatigue, nausea, anemia and cough.
|
Grades were obtained per CTCAE version 4.03. |
||||
|
† Grouped term for reactions with multiple preferred terms |
||||
|
a Diarrhea or colitis includes the preferred terms: colitis, enterocolitis, colitis microscopic, colitis ulcerative, diarrhea |
||||
|
b Pneumonia includes the preferred terms: All preferred term containing "pneumonia" except for "pneumonia aspiration"; bronchopneumonia, bronchopulmonary aspergillosis |
||||
|
c Rash includes the preferred terms: dermatitis (including allergic, exfoliative, perivascular), erythema (including multiforme), rash (including exfoliative, erythematous, follicular, generalized, macular & papular, pruritic, pustular), toxic skin eruption, drug eruption |
||||
|
d Transaminase elevation includes the preferred terms: alanine aminotransferase increased, aspartate aminotransferase increased, transaminases increased, hepatotoxicity |
||||
| Adverse Reactions
| COPIKTRA
N = 158 | Ofatumumab
N = 155 |
||
| Any Grade (%)
| Grade ≥ 3 (%)
| Any Grade (%)
| Grade ≥ 3 (%)
|
|
| Gastrointestinal disorders
| ||||
| Diarrhea or colitis †a
Nausea † Constipation Abdominal pain Vomiting | 57 23 17 16 15 | 25 0 <1 3 0 | 14 11 8 7 7 | 2 0 0 0 0 |
| General disorders and administration site conditions
| ||||
| Pyrexia Fatigue † | 29 25 | 3 4 | 10 23 | <1 4 |
| Hepatobiliary disorders
| ||||
| Transaminase elevation †d
| 11 | 6 | 4 | <1 |
| Infections and infestations
| ||||
| Upper respiratory tract infection †
Pneumonia †b Lower respiratory tract infection † | 28 27 18 | 0 22 4 | 16 8 10 | <1 3 1 |
| Investigations
| ||||
| Weight decreased | 11 | 0 | 2 | 0 |
| Metabolism and nutrition disorders
| ||||
| Decreased appetite Edema † | 13 11 | 0 1 | 3 5 | <1 0 |
| Musculoskeletal and connective tissue disorders
| ||||
| Musculoskeletal pain †
| 17 | 1 | 12 | <1 |
| Respiratory, thoracic and mediastinal disorders
| ||||
| Cough †
| 23 | 1 | 16 | 0 |
| Dyspnea | 12 | 3 | 7 | 0 |
| Skin and subcutaneous tissue disorders
| ||||
| Rash †c
| 27 | 11 | 15 | <1 |
|
Grades were obtained per CTCAE version 4.03. |
||||
| Laboratory Parameter
| COPIKTRA
N = 158 | Ofatumumab
N = 155 |
||
| Any Grade (%)
| Grade ≥ 3 (%)
| Any Grade (%)
| Grade ≥ 3 (%)
|
|
| Hematology abnormalities
| ||||
| Neutropenia Anemia Thrombocytopenia Lymphocytosis | 67 55 43 30 | 49 20 16 22 | 52 36 34 11 | 37 7 8 6 |
| Chemistry abnormalities
| ||||
| ALT increased Lipase increased AST increased Phosphate decreased Hyperkalemia Hyponatremia Amylase increased Hypoalbuminemia Creatinine increased Alkaline phosphatase increased Hypocalcemia Hypokalemia | 42 37 36 34 31 31 31 31 29 27 25 20 | 7 12 3 3 4 7 5 2 1 0 1 8 | 12 15 14 20 24 18 10 15 31 14 17 8 | 0 3 1 3 1 3 1 1 0 0 1 0 |
The data above are not an adequate basis for comparison of rates between the study drug and the active control.
7. Drug Interactions
7.1 Effects of Other Drugs on COPIKTRA
Coadministration with a strong CYP3A4 inhibitor increases duvelisib AUC [see Clinical Pharmacology (12.3)], which may increase the risk of COPIKTRA toxicities. Reduce COPIKTRA dosage when co-administered with a strong CYP3A4 inhibitor [see Dosage and Administration (2.4)].
Strong and Moderate CYP3A4 Inducers
Coadministration with a strong or moderate CYP3A4 inducer decreases duvelisib area under the curve (AUC) [see Clinical Pharmacology (12.3)], which may reduce COPIKTRA efficacy. Avoid coadministration of strong or moderate CYP3A4 inducers with COPIKTRA. If coadministration with a moderate CYP3A4 inducer cannot be avoided, increase the COPIKTRA dose. [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
7.2 Effects of COPIKTRA on Other Drugs
Coadministration with COPIKTRA increases AUC of a sensitive CYP3A4 substrate [see Clinical Pharmacology (12.3)] which may increase the risk of toxicities of these drugs. Consider reducing the dose of the sensitive CYP3A4 substrate and monitor for signs of toxicities of the co-administered sensitive CYP3A4 substrate.
8. Use In Specific Populations
8.1 Pregnancy
Based on findings from animal studies and the mechanism of action, COPIKTRA can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)].
There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of duvelisib to pregnant rats and rabbits during organogenesis caused adverse developmental outcomes including embryo-fetal mortality (resorptions, post-implantation loss, and decreased viable fetuses), alterations to growth (lower fetal weights) and structural abnormalities (malformations) at maternal doses 10 times and 39 times the MRHD of 25 mg BID in rats and rabbits, respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study in rats, pregnant animals received daily oral doses of duvelisib of 0, 10, 50, 150 and 275 mg/kg/day during the period of organogenesis. Administration of duvelisib at doses ≥ 50 mg/kg/day resulted in adverse developmental outcomes including reduced fetal weights and external abnormalities (bent tail and fetal anasarca), and doses ≥ 150 mg/kg/day resulted in maternal toxicity including mortality and no live fetuses (100% resorption) in surviving dams. In another study in pregnant rats receiving oral doses of duvelisib up to 35 mg/kg/day during the period of organogenesis, no maternal or embryo-fetal effects were observed. The dose of 50 mg/kg/day in rats is approximately 10 -times the MRHD of 25 mg BID.
In an embryo-fetal development study in rabbits, pregnant animals received daily oral doses of duvelisib of 0, 25, 100, and 200 mg/kg/day during the period of organogenesis. Administration of duvelisib at doses ≥ 100 mg/kg/day resulted in maternal toxicity (body weight losses or lower mean body weights and increased mortality) and adverse developmental outcomes (increased resorptions and post-implantation loss, abortion, and decreased numbers of viable fetuses). In another study in pregnant rabbits receiving oral doses of duvelisib up to 75 mg/kg/day, no maternal or embryo-fetal effects were observed. The dose of 100 mg/kg/day in rabbits is approximately 39 times the MRHD of 25 mg BID.
8.2 Lactation
There are no data on the presence of duvelisib and/or its metabolites in human milk, the effects on the breastfed child, or on milk production. Because of the potential for serious adverse reactions from duvelisib in a breastfed child, advise lactating women not to breastfeed while taking COPIKTRA and for 1 month after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Conduct pregnancy testing before initiation of COPIKTRA treatment.
Contraception
Females
Based on animal studies, COPIKTRA can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with COPIKTRA and for 1 month after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with COPIKTRA and for 1 month after the last dose.
Infertility
Based on testicular findings in animals, male fertility may be impaired by treatment with COPIKTRA [see Nonclinical Toxicology (13.1)]. There are no data on the effect of COPIKTRA on human fertility.
11. Copiktra Description
COPIKTRA (duvelisib) is a kinase inhibitor.
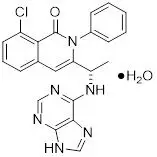
Duvelisib is a white-to-off-white crystalline solid with the empirical formula C22H17ClN6O•H2O and a molecular weight of 434.88 g/mol. Hydration can vary with relative humidity. Duvelisib contains a single chiral center as (S) enantiomer. Duvelisib is soluble in ethanol and practically insoluble in water. Duvelisib is described chemically as a hydrate of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one and has the following chemical structure:
12. Copiktra - Clinical Pharmacology
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of multiple doses of COPIKTRA 25 and 75 mg BID on the QTc interval was evaluated in patients with previously treated hematologic malignancies. Increases of > 20 ms in the QTc interval were not observed.
12.3 Pharmacokinetics
At steady state following 25 mg BID administration of duvelisib in patients, the geometric mean (CV%) maximum concentration (Cmax) was 1.5 (64%) µg/mL and AUC was 7.9 (77%) µg•h/mL.
Absorption
The absolute bioavailability of 25 mg duvelisib after a single oral dose in healthy volunteers was 42%. The median time to peak concentration (Tmax) was observed at 1 to 2 hours in patients.
Effect of Food
COPIKTRA may be administered without regard to food. The administration of a single dose of COPIKTRA with a high-fat meal (fat accounted for approximately 50% of the total caloric content of the meal) decreased Cmax by approximately 37% and decreased the AUC by approximately 6%, relative to fasting conditions.
Distribution
Protein binding of duvelisib is greater than 98% with no concentration dependence. The mean blood-to-plasma ratio was 0.5. The geometric mean (CV%) apparent volume of distribution at steady state (Vss/F) is 28.5 L (62%). Duvelisib is a substrate of P-glycoprotein (P-gp) and BCRP in vitro.
Elimination
The geometric mean (CV%) apparent systemic clearance at steady-state is 4.2 L/hr (56%) in patients with lymphoma or leukemia. The geometric mean (CV%) terminal elimination half-life of duvelisib is 4.7 hours (57%).
Metabolism
Duvelisib is primarily metabolized by cytochrome P450 CYP3A4.
Excretion
Following a single 25 mg oral dose of radiolabeled duvelisib, 79% of the radioactivity was excreted in feces (11% unchanged) and 14% was excreted in the urine (< 1% unchanged).
Specific Populations
Age (18 to 90 years), sex, race, renal impairment (creatinine clearance 23 to 80 mL/ min), hepatic impairment (Child Pugh Class A, B, and C) and body weight (40 to 154 kg) had no clinically significant effect on the exposure of duvelisib.
Drug Interaction Studies
CYP3A4 Inhibitors
Coadministration of a single COPIKTRA 10 mg dose with ketoconazole (strong CYP3A4 inhibitor) at 200 mg BID for 5 days in healthy adults increased duvelisib Cmax by 1.7-fold and AUC by 4-fold. Based on physiologically-based pharmacokinetic (PBPK) modeling and simulation, the increase in duvelisib exposure at steady state is estimated to be ~2-fold when coadministered with strong CYP3A4 inhibitors [see Dosage and Administration (2.4) and Drug Interactions (7.1)]. PBPK modeling and simulation estimated no effect on duvelisib exposures from concomitantly used mild or moderate CYP3A4 inhibitors.
Strong and Moderate CYP3A4 Inducers
Coadministration of a single COPIKTRA 25 mg dose with rifampin (strong CYP3A4 inducer) 600 mg once daily for 7 days in healthy adults decreased duvelisib Cmax by 66% and AUC by 82% [see Dosage and Administration (2.5) and Drug Interactions (7.1)].
Co-administration of etravirine (moderate CYP3A4 inducer) 200 mg twice daily of etravirine for 12 days with a single COPIKTRA 25 mg dose in healthy adults decreased duvelisib Cmax by 16% and AUC by 35%. [see Dosage and Administration (2.5) and Drug Interactions (7.1)].
CYP3A4 Substrates
Coadministration of multiple doses of COPIKTRA 25 mg BID for 5 days with a single midazolam (sensitive CYP3A4 substrate) 2 mg dose in healthy adults increased the midazolam AUC by 4.3-fold and Cmax by 2.2-fold [see Drug Interactions (7.2)].
In Vitro Studies
Duvelisib is a substrate of P-glycoprotein (P-gp) and breast cancer-resistant protein (BCRP).
Duvelisib does not inhibit OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, BCRP, or P-gp.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with duvelisib.
Duvelisib did not cause genetic damage in in vitro or in vivo assays.
Fertility studies with duvelisib were not conducted. Histological findings in male and female rats were observed in the repeat dose toxicity studies and included testis (seminiferous epithelial atrophy, decreased weight, soft testes), and epididymis (small size, oligo/aspermia) in males and ovary (decreased weight) and uterus (atrophy) in females.
14. Clinical Studies
A randomized, multicenter, open-label trial (Study 1; NCT02004522) compared COPIKTRA versus ofatumumab in 319 adult patients with CLL (N = 312) or SLL (N = 7) after at least one prior therapy. The trial excluded patients with prior autologous transplant within 6 months or allogeneic transplant, prior exposure to a PI3K inhibitor or a Bruton's tyrosine kinase (BTK) inhibitor. The trial required hepatic transaminases ≤ 3 times upper limit of normal (ULN), total bilirubin ≤ 1.5 times ULN, and serum creatinine ≤ 2 times ULN.
The study randomized patients with a 1:1 ratio to receive either COPIKTRA 25 mg BID until disease progression or unacceptable toxicity or ofatumumab for 7 cycles. Ofatumumab was administered intravenously at an initial dose of 300 mg, followed one week later by 2000 mg once weekly for 7 doses, and then 2000 mg once every 4 weeks for 4 additional doses.
The approval of COPIKTRA was based on efficacy and safety analysis of patients with at least 2 prior lines of therapy, where the benefit:risk appeared greater in this more heavily pretreated population compared to the overall trial population.
In this subset (95 randomized to COPIKTRA, 101 to ofatumumab), the median patient age was 69 years (range: 40 to 90 years), 59% were male, and 88% had an ECOG performance status of 0 or 1. Forty-six percent received 2 prior lines of therapy, and 54% received 3 or more prior lines. At baseline, 52% of patients had at least one tumor ≥ 5 cm, and 22% of patients had a documented 17p deletion.
During randomized treatment, the median duration of exposure to COPIKTRA was 13 months (range: 0.2 to 37), with 80% of patients receiving at least 6 months and 52% receiving at least 12 months of COPIKTRA. The median duration of exposure to ofatumumab was 5 months (range: < 0.1 to 6).
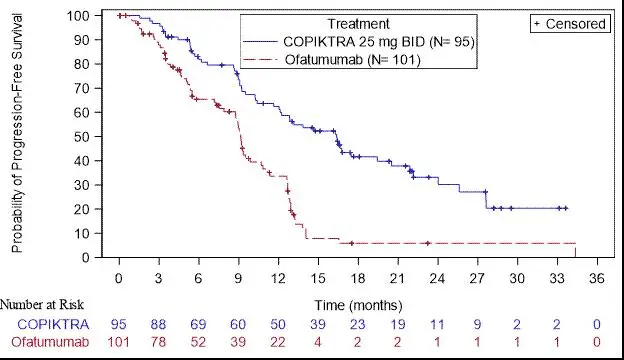
Efficacy was based on progression-free survival (PFS) as assessed by an Independent Review Committee (IRC). Other efficacy measures included overall response rate. Efficacy of COPIKTRA compared to ofatumumab specifically in patients treated with at least two prior therapies is presented in Table 8 and Figure 1.
|
Abbreviations: CI = confidence interval; CR = complete response; IRC = Independent Review Committee; PFS = progression-free survival; PR = partial response; SE = standard error |
||
|
a Kaplan-Meier estimate |
||
|
b Standard Error of ln(hazard ratio) = 0.2 |
||
|
c IWCLL or revised IWG response criteria, with modification for treatment-related lymphocytosis |
||
| Outcome per IRC
| COPIKTRA
N = 95 | Ofatumumab
N = 101 |
| PFS
|
||
| Number of events, n (%) | 55 (58) | 70 (69) |
| Progressive disease | 44 | 62 |
| Death | 11 | 8 |
| Median PFS (SE), months a
| 16.4 (2.1) | 9.1 (0.5) |
| Hazard Ratio (SE), b COPIKTRA/ofatumumab | 0.40 (0.2) |
|
| Response rate
|
||
| ORR, n (%)c
| 74 (78) | 39 (39) |
| CR | 0 (0) | 0 (0) |
| PR | 74 (78) | 39 (39) |
| Difference in ORR, % (SE) | 39 (6.4) |
|
Figure 1. Kaplan-Meier Curve of PFS per IRC In Patients with at Least 2 Prior Therapies (Study 1)
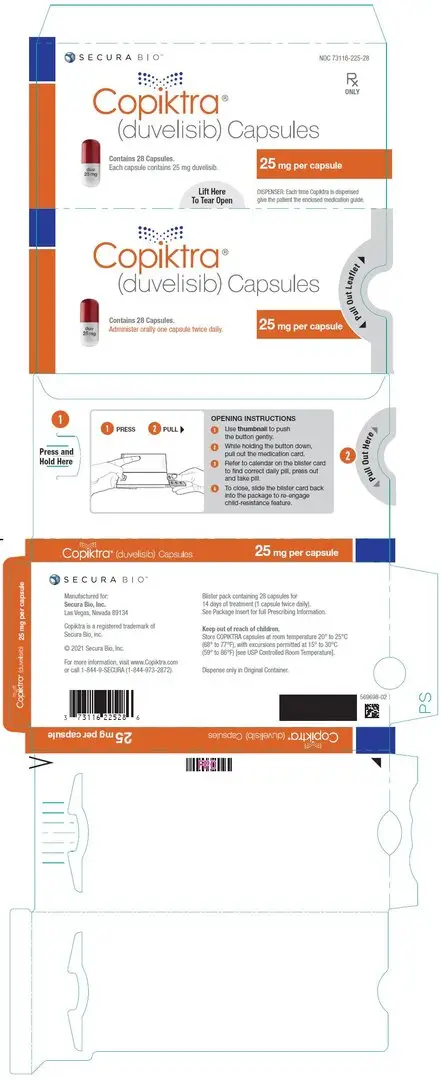
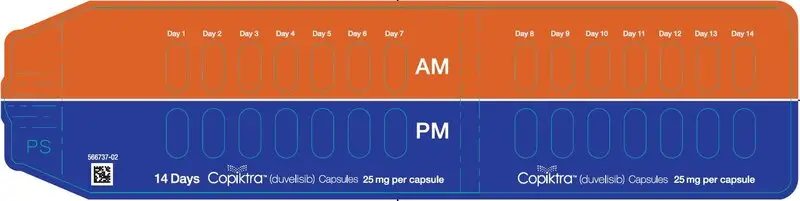
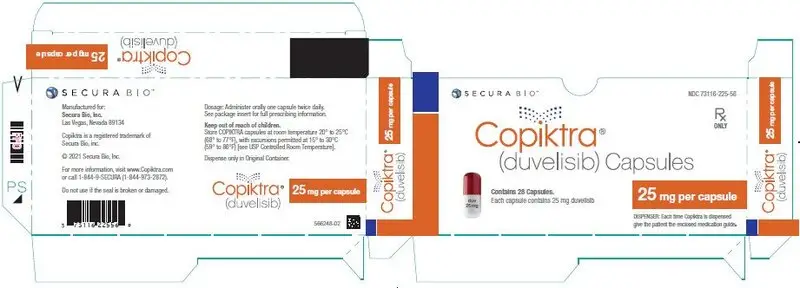
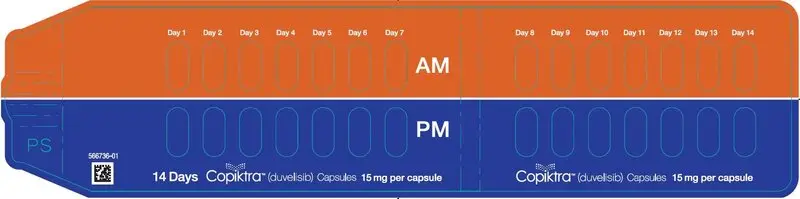
16. How is Copiktra supplied
|
Abbreviations: HDPE = high-density polyethylene; NDC = National Drug Code; No. = number |
|||
| Capsule Strength
| Description
| Package Configuration
| NDC No.
|
| 25 mg | White to off-white and Swedish orange opaque capsules marked with "duv 25 mg" in black ink |
|
|
| 15 mg | Pink opaque capsules marked with "duv 15 mg" in black ink |
|
|
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Physicians and healthcare professionals are advised to discuss the following with patients prior to treatment with COPIKTRA:
●Infections
●Diarrhea or Colitis
●Cutaneous Reactions
●Pneumonitis
●Hepatotoxicity
●Neutropenia
●Embryo-Fetal Toxicity
Advise females of reproductive potential to use effective contraception during treatment and for 1 month after receiving the last dose of COPIKTRA [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1, 8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with COPIKTRA and for 1 month after the last dose [see Warnings and Precautions (5.7) and Use in Specific Populations (8.1, 8.3)].
●Lactation
●Instructions for Taking COPIKTRA
Advise patients that if a dose is missed by fewer than 6 hours, to take the missed dose right away and take the next dose as usual. If a dose is missed by more than 6 hours, advise patients to wait and take the next dose at the usual time [see Dosage and Administration (2.3)].
Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products, before and during treatment with COPIKTRA [see Drug Interactions (7)].
Distributed by:
Secura Bio, Inc.
1995 Village Center Circle, Suite 128
Las Vegas, NV 89134
© Secura Bio
USCPR2007403
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: 12/2021 |
|
|
MEDICATION GUIDE COPIKTRA™ (co-PIK-trah)
|
||
|
What is the most important information I should know about COPIKTRA? COPIKTRA can cause serious side effects, including:
Your healthcare provider may need to prescribe medicines, including a steroid medicine, to help treat your skin rash or other skin reactions.
If you have any of the above serious side effects during treatment with COPIKTRA, your healthcare provider may stop your treatment for a period of time, change your dose of COPIKTRA, or completely stop your treatment with COPIKTRA. See “See "What are the possible side effects of COPIKTRA?" ” for more information about side effects. |
||
|
What is COPIKTRA? COPIKTRA is a prescription medicine used to treat adults with:
It is not known if COPIKTRA is safe and effective in children less than 18 years of age. |
||
|
What should I tell my healthcare provider before taking COPIKTRA? Before taking COPIKTRA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. COPIKTRA and certain other medicines may affect each other. |
||
|
How should I take COPIKTRA?
|
||
|
What are possible side effects of COPIKTRA? COPIKTRA may cause serious side effects, including:
Common side effects of COPIKTRA include: |
||
|
|
|
|
These are not all the possible side effects of COPIKTRA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store COPIKTRA?
Keep COPIKTRA and all medicines out of the reach of children. |
||
|
General information about the safe and effective use of COPIKTRA: Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use COPIKTRA for a condition for which it was not prescribed. Do not give COPIKTRA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about COPIKTRA that is written for health professionals. |
||
|
What are the ingredients in COPIKTRA?
Distributed by: Secura Bio, Inc. 1995 Village Center Circle, Suite 128, Las Vegas, NV 89134 © Secura Bio USCPR2008002 For more information , go to www.Copiktra.com or call on 1-844-9-SECURA (1-844973-2872) |
||
| COPIKTRA
duvelisib capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| COPIKTRA
duvelisib capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Secura Bio, Inc. (117026236) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Curia Global, Inc. | 124193793 | ANALYSIS(73116-225, 73116-215) , API MANUFACTURE(73116-225, 73116-215) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent CTS, LLC | 962674474 | ANALYSIS(73116-225, 73116-215) , MANUFACTURE(73116-225, 73116-215) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Packaging Coordinators, Inc. | 053217022 | LABEL(73116-225, 73116-215) , PACK(73116-225, 73116-215) | |