Drug Detail:Cotempla xr-odt (Methylphenidate)
Drug Class: CNS stimulants
Highlights of Prescribing Information
COTEMPLA XR-ODT (methylphenidate extended-release orally disintegrating tablets), CII
Initial U.S. Approval: 1955
WARNING: ABUSE AND DEPENDENCE
See full prescribing information for complete boxed warning.
- CNS stimulants, including COTEMPLA XR-ODT, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence ( 5.1, 9.2, 9.3)
- Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy ( 5.1, 9.2)
Indications and Usage for Cotempla XR-ODT
COTEMPLA XR-ODT is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in pediatric patients 6 to 17 years of age. ( 1)
Cotempla XR-ODT Dosage and Administration
- Recommended starting dose for pediatric patients 6 to 17 years of age is 17.3 mg given orally once daily in the morning. Dosage may be increased weekly in increments of 8.6 mg to 17.3 mg per day. Daily dosage above 51.8 mg is not recommended. ( 2.2)
- Patients are advised to take COTEMPLA XR-ODT consistently either with food or without food. ( 2.2)
Dosage Forms and Strengths
Extended-Release Orally Disintegrating Tablets: 8.6 mg, 17.3 mg and 25.9 mg. ( 3)
Contraindications
- Known hypersensitivity to methylphenidate or product components. ( 4)
- Concurrent treatment with a monoamine oxidase inhibitor (MAOI), or use of an MAOI within the preceding 14 days. ( 4)
Warnings and Precautions
- Serious Cardiovascular Reactions: Sudden death has been reported in association with CNS stimulants at recommended doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. In adults, sudden death, stroke, and myocardial infarction have been reported. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart arrhythmias, or coronary artery disease. ( 5.2)
- Blood Pressure and Heart Rate Increases: Monitor blood pressure and pulse. Consider the benefits and risks in patients for whom an increase in blood pressure or heart rate would be problematic. ( 5.3)
- Psychiatric Adverse Reactions: Use of stimulants may cause psychotic or manic symptoms in patients with no prior history, or exacerbation of symptoms in patients with pre-existing psychiatric illness. Evaluate for bipolar disorder prior to COTEMPLA XR-ODT use. ( 5.4)
- Priapism: Cases of painful and prolonged penile erections and priapism have been reported with methylphenidate products. Immediate medical attention should be sought if signs or symptoms or prolonged penile erections or priapism are observed. ( 5.5)
- Peripheral Vasculopathy, including Raynaud's Phenomenon: Stimulants used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Careful observation for digital changes is necessary during treatment with ADHD stimulants. ( 5.6)
- Long-term Suppression of Growth: Monitor height and weight at appropriate intervals in pediatric patients. ( 5.7)
Adverse Reactions/Side Effects
Based on accumulated data from other methylphenidate products, the most common (>5% and twice the rate of placebo) adverse reactions are appetite decreased, insomnia, nausea, vomiting, dyspepsia, abdominal pain, weight decreased, anxiety, dizziness, irritability, affect lability, tachycardia, and blood pressure increased. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Neos Therapeutics, Inc. at 1-888-319-1789 or http://www.COTEMPLAXRODT.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Antihypertensive drugs: Monitor blood pressure. Adjust dosage of antihypertensive drug as needed. ( 7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2021
Related/similar drugs
Adderall, Vyvanse, methylphenidate, Strattera, Ritalin, ConcertaFull Prescribing Information
WARNING: ABUSE AND DEPENDENCE
CNS stimulants, including COTEMPLA XR-ODT, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3)] .
1. Indications and Usage for Cotempla XR-ODT
COTEMPLA XR-ODT is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in pediatric patients 6 to 17 years of age [see Clinical Studies (14)].
2. Cotempla XR-ODT Dosage and Administration
2.1 Pretreatment Screening
Prior to initiating treatment with COTEMPLA XR-ODT, assess for the presence of cardiac disease (i.e. perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2)] .
Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy. Maintain careful prescription records, educate patients about abuse, and periodically re-evaluate the need for COTEMPLA XR-ODT use [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9)].
2.2 General Dosing Information
COTEMPLA XR-ODT is given orally once daily in the morning.
Advise patients to take COTEMPLA XR-ODT consistently either with food or without food [see Clinical Pharmacology (12.3)] .
The recommended starting dose of COTEMPLA XR-ODT for patients 6 to 17 years of age is 17.3 mg once daily in the morning. The dose may be titrated weekly in increments of 8.6 mg to 17.3 mg. Daily doses above 51.8 mg have not been studied and are not recommended.
The dose should be individualized according to the needs and responses of the patient.
Pharmacological treatment of ADHD may be needed for extended periods. Periodically re-evaluate the long-term use of COTEMPLA XR-ODT and adjust dosage as needed.
2.3 Dose Reduction and Discontinuation
If paradoxical aggravation of symptoms or other adverse effects occur, reduce dosage, or, if necessary, discontinue the drug. COTEMPLA XR-ODT should be periodically discontinued to assess the child's condition. If improvement is not observed after appropriate dosage adjustment over a one-month period, discontinue the drug.
2.4 COTEMPLA XR-ODT Administration
Instruct the patient or caregiver on the following administration instructions:
- Do not remove the tablet from the blister pack until just prior to dosing. Take the tablet immediately after opening the blister pack. Do not store the tablet for future use.
- Use dry hands when opening the blister pack.
- Remove the tablet by peeling back the foil on the blister pack. Do not push the tablet through the foil.
- As soon as the blister is opened, remove the tablet and place on the patient's tongue.
- Place the whole tablet on the tongue and allow it to disintegrate without chewing or crushing.
- The tablet will disintegrate in saliva so that it can be swallowed. No liquid is needed to take the tablet.
3. Dosage Forms and Strengths
- 8.6 mg Extended-Release Orally Disintegrating Tablet: round, purple to light purple mottled (debossed "T1" on one side and plain on the other)
- 17.3 mg Extended-Release Orally Disintegrating Tablet: round, purple to light purple mottled (debossed "T2" on one side and plain on the other)
- 25.9 mg Extended-Release Orally Disintegrating Tablet: round, purple to light purple mottled (debossed "T3" on one side and plain on the other)
4. Contraindications
COTEMPLA XR-ODT is contraindicated in patients with:
- Known hypersensitivity to methylphenidate or other components of COTEMPLA XR-ODT. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with methylphenidate products [see Adverse Reactions (6.2)] .
- Concomitant treatment with monoamine oxidase inhibitors (MAOIs), and also within a minimum of 14 days following discontinuation of treatment with a monoamine oxidase inhibitor because of the risk of hypertensive crisis [see Drug Interactions (7.1)] .
5. Warnings and Precautions
5.1 Potential for Abuse and Dependence
CNS stimulants, including COTEMPLA XR-ODT, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy [see Boxed Warning and Drug Abuse and Dependence (9.2, 9.3)] .
5.2 Serious Cardiovascular Reactions
Sudden death, stroke and myocardial infarction have occurred in adults treated with CNS stimulants at recommended doses. Sudden death has occurred in pediatric patients with structural cardiac abnormalities and other serious cardiac problems taking CNS stimulants at recommended doses for ADHD. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, and other serious heart problems. Perform further evaluation on patients who develop exertional chest pain, unexplained syncope, or arrhythmias during COTEMPLA XR-ODT treatment.
5.3 Blood Pressure and Heart Rate Increases
CNS stimulants cause an increase in blood pressure (mean increase approximately 2 to 4 mm Hg) and heart rate (mean increase approximately 3 to 6 bpm). Individuals may have larger increases. Monitor all patients for hypertension and tachycardia.
5.4 Psychiatric Adverse Reactions
Exacerbation of Pre-Existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixed episode in patients. Prior to initiating treatment, screen patients for risk factors for developing a manic episode (e.g. comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
New Psychotic or Manic Symptoms
CNS stimulants, at recommended doses, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking or mania) in patients without a prior history of psychotic illness or mania. If such symptoms occur, consider discontinuing COTEMPLA XR-ODT. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients, compared to 0 in placebo-treated patients.
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate products in both pediatric and adult patients. Priapism was not reported with drug initiation but developed after some time on the drug, often subsequent to an increase in dose. Priapism has also appeared during a period of drug withdrawal (drug holidays or during discontinuation). Patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, including Raynaud's Phenomenon
CNS stimulants, including COTEMPLA XR-ODT, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
5.7 Long-Term Suppression of Growth
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in pediatric patients ages 7 to 10 years who were randomized to either methylphenidate or nonmedication-treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and nonmedication-treated pediatric patients over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e. treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development.
Closely monitor growth (weight and height) in pediatric patients treated with CNS stimulants, including COTEMPLA XR-ODT. Patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
6. Adverse Reactions/Side Effects
The following are discussed in more detail in other sections of the labeling:
- Known hypersensitivity to methylphenidate or other ingredients of Cotempla XR-ODT [see Contraindications (4)]
- Hypertensive crisis when used concomitantly with monoamine oxidase inhibitors [see Contraindications (4) and Drug Interactions (7.1)]
- Drug dependence [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9.2, 9.3)]
- Serious cardiovascular reactions [see Warnings and Precautions (5.2)]
- Blood pressure and heart rate increases [see Warnings and Precautions (5.3)]
- Psychiatric adverse reactions [see Warnings and Precautions (5.4)]
- Priapism [see Warnings and Precautions (5.5)]
- Peripheral vasculopathy, including Raynaud's phenomenon [see Warnings and Precautions (5.6)]
- Long-term suppression of growth [see Warnings and Precautions (5.7)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Clinical Trials Experience with Other Methylphenidate Products in Children, Adolescents, and Adults with ADHD
Commonly reported (≥2% of the methylphenidate group and at least twice the rate of the placebo group) adverse reactions from placebo-controlled trials of methylphenidate products include: appetite decreased, weight decreased, nausea, abdominal pain, dyspepsia, dry mouth, vomiting, insomnia, anxiety, nervousness, restlessness, affect lability, agitation, irritability, dizziness, vertigo, tremor, blurred vision, blood pressure increased, heart rate increased, tachycardia, palpitations, hyperhidrosis, and pyrexia.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of methylphenidate products. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are as follows:
Blood and Lymphatic System Disorders: Pancytopenia, Thrombocytopenia, Thrombocytopenic purpura
Cardiac Disorders: Angina pectoris, Bradycardia, Extrasystole, Supraventricular tachycardia, Ventricular extrasystole
Eye Disorders: Diplopia, Mydriasis, Visual impairment
General Disorders: Chest pain, Chest discomfort, Hyperpyrexia
Immune System Disorders: Hypersensitivity reactions such as Angioedema, Anaphylactic reactions, Auricular swelling, Bullous conditions, Exfoliative conditions, Urticarias, Pruritis NEC, Rashes, Eruptions, and Exanthemas NEC
Investigations: Alkaline phosphatase increased, Bilirubin increased, Hepatic enzyme increased, Platelet count decreased, White blood cell count abnormal
Musculoskeletal, Connective Tissue and Bone Disorders: Arthralgia, Myalgia, Muscle twitching, Rhabdomyolysis
Nervous System Disorders: Convulsion, Grand mal convulsion, Dyskinesia, Serotonin syndrome in combination with serotonergic drugs
Psychiatric Disorders: Disorientation, Hallucination, Hallucination auditory, Hallucination visual, Libido changes, Mania
Urogenital System: Priapism
Skin and Subcutaneous Tissue Disorders: Alopecia, Erythema
Vascular Disorders: Raynaud's phenomenon
7. Drug Interactions
7.1 Clinically Important Interactions with COTEMPLA XR-ODT
| Monoamine Oxidase Inhibitors (MAOI) | |
| Clinical Impact: | Concomitant use of MAOIs and CNS stimulants can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure [see Contraindications (4)] . |
| Intervention: | Do not administer COTEMPLA-XR ODT concomitantly with MAOIs or within 14 days after discontinuing MAOI treatment. |
| Examples: | selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue |
| Gastric pH Modulators | |
| Clinical Impact: | May change the release profile and alter the pharmacodynamics of COTEMPLA-XR ODT. |
| Intervention: | Concomitant use of Cotempla XR-ODT with a gastric pH modulator (i.e., a H2-blocker or a proton pump inhibitor) is not recommended. |
| Examples: | omeprazole, famotidine, sodium bicarbonate |
| Antihypertensive Drugs | |
| Clinical Impact: | Cotempla XR-ODT may decrease the effectiveness of drug used to treat hypertension [see Warnings and Precautions (5.3)]. |
| Intervention: | Monitor blood pressure and adjust the dosage of the antihypertensive drug as needed. |
| Examples: | Potassium-sparing and thiazide diuretics, calcium channel blockers, angiotensin-converting-enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta blockers, centrally acting alpha-2 receptor agonists |
| Risperidone | |
| Clinical Impact: | Combined use of methylphenidate with risperidone when there is a change, whether an increase or decrease, in dosage of either or both medications, may increase the risk of extrapyramidal symptoms (EPS). |
| Intervention: | Monitor for signs of EPS. |
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to COTEMPLA XR-ODT during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychostimulants at 1-866-961-2388.
Risk Summary
Published studies and postmarketing reports on methylphenidate use during pregnancy are insufficient to inform a drug-associated risk of adverse pregnancy-related outcomes [see Data] . There are risks to the fetus associated with the use of central nervous system (CNS) stimulants during pregnancy [see Clinical Considerations]. No teratogenic effects were observed in embryo-fetal development studies with oral administration of methylphenidate to pregnant rats and rabbits during organogenesis at doses 4 and 18 times, respectively, the maximum recommended human dose (MRHD) of 51.8 mg (as base). However, spina bifida was observed in rabbits at a dose 60 times the MRHD [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal adverse reactions
CNS stimulants, such as COTEMPLA XR-ODT, can cause vasoconstriction and thereby decrease placental perfusion. No fetal and/or neonatal adverse reactions have been reported with the use of therapeutic doses of methylphenidate during pregnancy; however, premature delivery and low birth weight infants have been reported in amphetamine-dependent mothers.
Data
Human Data
A limited number of pregnancies have been reported in published observational studies and postmarketing reports describing methylphenidate use during pregnancy. Due to the small number of methylphenidate-exposed pregnancies with known outcomes, these data cannot definitely establish or exclude any drug-associated risk during pregnancy. Methodological limitations of these observational studies include small sample size, concomitant use of other medications, lack of detail regarding dose and duration of exposure to methylphenidate and non-generalizability of the enrolled populations.
Animal Data
In studies conducted in rats and rabbits, methylphenidate was administered orally at doses of up to 75 and 200 mg/kg/day, respectively, during the period of organogenesis. Teratogenic effects (increased incidence of fetal spina bifida) were observed in rabbits at the highest dose, which is approximately 60 times the maximum recommended human dose (MRHD) of 51.8 mg (as base) for adolescents on a mg/m 2 basis. The no effect level for embryo-fetal development in rabbits was 60 mg/kg/day (18 times the MRHD for adolescent on a mg/m 2 basis). There was no evidence of specific teratogenic activity in rats, although increased incidences of fetal skeletal variations were seen at the highest dose level (11 times the MRHD on a mg/m 2 basis for adolescent), which was also maternally toxic. The no effect level for embryo-fetal development in rats was 25 mg/kg/day (4 times the MRHD on a mg/m 2 basis for adolescent).
8.2 Lactation
Risk Summary
Limited published literature, based on breast milk sampling from five mothers, reports that methylphenidate is present in human milk, which resulted in infant doses of 0.16% to 0.7% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.1 and 2.7. There are no reports of adverse effects on the breastfed infant and no effects on milk production. Long-term neurodevelopmental effects on infants from stimulant exposure are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for COTEMPLA XR-ODT and any potential adverse effects on the breastfed child from COTEMPLA XR-ODT or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of COTEMPLA XR-ODT have been established in pediatric patients 6 to 17 years of age in one adequate and well-controlled study in pediatric patients 6 to 12 years, pharmacokinetic data in adolescents, and safety information from other methyphenidate-containing products [see Clinical Pharmacology (12) and Clinical Studies (14)] .
The long-term efficacy of methylphenidate in pediatric patients has not been established. Safety and effectiveness of COTEMPLA XR-ODT in pediatric patients below 6 years of age have not been established.
Long Term Suppression Growth
Growth should be monitored during treatment with stimulants, including COTEMPLA XR-ODT. Children who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.7)] .
Juvenile Animal Toxicity Data
Rats treated with methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only. The doses at which these findings were observed are at least 6 times the maximum recommended human dose (MRHD) of 51.8 mg (as base) for pediatric patients on a mg/m 2 basis.
In the study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (postnatal day 7) and continuing through sexual maturity (postnatal week 10). When these animals were tested as adults (postnatal weeks 13-14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day [approximately 6 times the MRHD of 51.8 mg (as base) on a mg/m 2 basis] or greater, and a deficit in the acquisition of a specific learning task was observed in females exposed to the highest dose (12 times the MRHD on a mg/m 2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (half the MRHD on a mg/m 2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
9. Drug Abuse and Dependence
9.1 Controlled Substance
COTEMPLA XR-ODT contains methylphenidate, a Schedule II controlled substance.
9.2 Abuse
CNS stimulants including COTEMPLA XR-ODT, other methylphenidate-containing products, and amphetamines have a high potential for abuse. Abuse is characterized by impaired control over drug use, compulsive use, continued use despite harm, and craving.
Signs and symptoms of CNS stimulant abuse include increased heart rate, respiratory rate, blood pressure, and/or sweating, dilated pupils, hyperactivity, restlessness, insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and/or abdominal pain. Anxiety, psychosis, hostility, aggression, suicidal or homicidal ideation have also been observed. Abusers of CNS stimulants may chew, snort, inject, or use other unapproved routes of administration which can result in overdose and death [see Overdosage (10)] .
To reduce the abuse of CNS stimulants including COTEMPLA XR-ODT, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records educate patients and their families about abuse and on proper storage and disposal of CNS stimulants [see How Supplied/Storage and Handling (16)] , monitor for signs of abuse while on therapy, and re-evaluate the need for COTEMPLA XR-ODT use.
9.3 Dependence
Tolerance
Tolerance (a state of adaptation in which exposure to a drug results in a reduction of the drug's desired and/or undesired effects over time) can occur during chronic therapy with CNS stimulants including COTEMPLA XR-ODT.
Dependence
Physical dependence (a state of adaptation manifested by a withdrawal syndrome produced by abrupt cessation, rapid dose reduction, or administration of an antagonist) can occur in patients treated with CNS stimulants including COTEMPLA XR-ODT. Withdrawal symptoms after abrupt cessation following prolonged high-dosage administration of CNS stimulants include dysphoric mood; depression, fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
10. Overdosage
10.1 Signs and Symptoms
Signs and symptoms of acute methylphenidate overdosage, resulting principally from overstimulation of the CNS and from excessive sympathomimetic effects, may include the following: nausea, vomiting, diarrhea, restlessness, anxiety, agitation, tremors, hyperflexia, muscle twitching, convulsion (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, hypotension, tachypnea, mydriasis, dryness of mucous membranes, and rhabdomyolysis.
10.2 Management of Overdose
Consult with a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice on the management of overdosage with methylphenidate. Provide supportive care, including close medical supervision and monitoring. Treatment should consist of general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdosage. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. Use supportive and symptomatic measures.
11. Cotempla XR-ODT Description
COTEMPLA XR-ODT contains methylphenidate, a central nervous system (CNS) stimulant. COTEMPLA XR-ODT is an extended-release orally disintegrating tablet intended for once daily administration. COTEMPLA XR-ODT contains approximately 25% immediate-release and 75% extended-release methylphenidate. Methylphenidate is ionically-bound to the sulfonate of polystyrene sulfonate particles.
COTEMPLA XR-ODT contains 8.6 mg, 17.3 mg or 25.9 mg of methylphenidate which is the same as the amount of methylphenidate (base equivalent) found, respectively, in 10 mg, 20 mg and 30 mg strength methylphenidate hydrochloride products.
The chemical name of methylphenidate is methyl α-phenyl-2-piperidineacetate, and its structural formula is shown in Figure 1.
Figure 1: Methylphenidate Structure
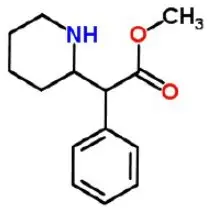
C 14H 19NO 2 Mol. Wt. 233.31
COTEMPLA XR-ODT also contains the following inactive ingredients: Mannitol, Fructose, Microcrystalline Cellulose, Crospovidone, Methacrylic Acid, Polystyrene Sulfonate, Citric Acid, Colloidal Silicon Dioxide, Grape Flavor, Natural Masking Type Powder, Triethyl Citrate, Magnesium Stearate, Ethylcellulose, Sucralose, Lake Blend Purple, and Polyethylene Glycol.
12. Cotempla XR-ODT - Clinical Pharmacology
12.1 Mechanism of Action
Methylphenidate is a central nervous system (CNS) stimulant. The mode of therapeutic action in ADHD is not known.
12.2 Pharmacodynamics
Methylphenidate is a racemic mixture comprised of the d- and 1-isomers. The d-isomer is more pharmacologically active than the l-isomer. Methylphenidate is thought to block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
12.3 Pharmacokinetics
After oral administration of COTEMPLA XR-ODT, circulation levels of l- methylphenidate (MPH) were about 2% of total MPH.
Absorption
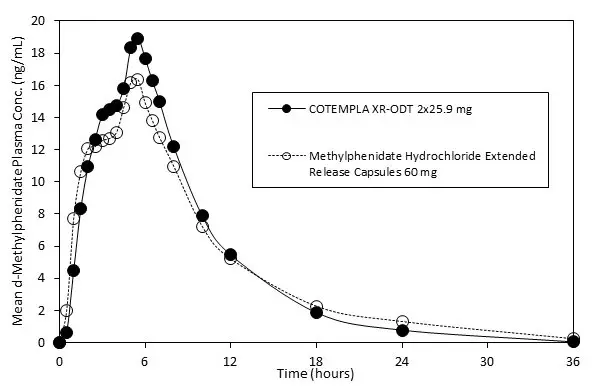
Following a single dose of 51.8 mg (2×25.9 mg daily) COTEMPLA XR-ODT in healthy adult subjects under fasted conditions, plasma methylphenidate (MPH) reached maximal concentration (C max) at a median time of 5 hours after dosing. Compared to an extended release capsule formulation of methylphenidate, methylphenidate mean C max and exposure (AUC inf) was about 26% and 6% higher, respectively, after COTEMPLA XR-ODT administration (Figure 2).
Figure 2: Mean d-Methylphenidate Plasma Concentration-Time Profiles After Administration of COTEMPLA XR-ODT or Methylphenidate Hydrochloride Extended-Release Capsule in Healthy Volunteers Under Fasted Conditions
Elimination
Plasma methylphenidate concentrations decline monophasically following oral administration of COTEMPLA XR-ODT. The mean plasma terminal elimination half-life of methylphenidate was about 4 hours in healthy volunteers following a single 51.8 mg dose administration.
Specific Populations
Male and Female Patients and Ethnic Groups
There is insufficient experience with the use of COTEMPLA XR-ODT to detect gender or ethnic variations in pharmacokinetics.
Pediatric Patients
The pharmacokinetics of methylphenidate after COTEMPLA XR-ODT administration were studied in pediatric patients (6-17 years of age) with ADHD under fasted conditions. After a single oral dose of 51.8 mg COTEMPLA XR-ODT, plasma concentrations of methylphenidate in children (6-12 years of age) were approximately twice the concentrations observed in adults. Exposure levels in adolescent patients (13 -17 years of age) were similar to those in adults. Body weight normalized clearance values were similar across the age groups (Table 2).
| PK Parameter | Children (n=24) | Adolescent (n=8) | Adult (n=38) |
|---|---|---|---|
| T max (hr) † | 4.6 (2.0-8.0) | 5.31 (3.5-8.0) | 4.98 (2.5 – 6.5) |
| T ½ (hr) | 4.43±1.0 | 3.93±0.33 | 4.00±0.73 |
| C max (ng/mL) | 32.7±9.83 | 20.2±5.79 | 20.8±5.22 |
| Cl (L/hr/kg) | 6.21±1.48 | 5.54±1.19 | 5.48±1.46 |
| AUC ∞ (hr*ng/mL) | 328.9±90.21 | 187.2±62.05 | 169.1±57.13 |
| † data presented as median range | |||
Patients with Renal Impairment
There is no experience with the use of COTEMPLA XR-ODT in patients with renal insufficiency. After oral administration of radiolabeled methylphenidate in humans, methylphenidate was extensively metabolized and approximately 80% of the radioactivity was excreted in the urine in the form of PPAA. Since renal clearance is not an important route of methylphenidate clearance, renal insufficiency is expected to have little effect on the pharmacokinetics of COTEMPLA XR-ODT.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas and, in males only, an increase in hepatoblastomas at a daily dose of approximately 60 mg/kg/day. For pediatric patients, this dose is approximately 4 times the maximum recommended human dose of 51.8 (as base) on a mg/m 2 basis. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown.
Methylphenidate did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 5 times the maximum recommended dose of 51.8 mg (as base) for pediatric patients on a mg/m 2 basis.
Mutagenesis
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay or the in vitro mouse lymphoma cell forward mutation assay. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary (CHO) cells. Methylphenidate was negative in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week Continuous Breeding study. The study was conducted at doses up to 160 mg/kg/day, approximately 12-fold the maximum recommended human dose of 51.8 (as base) for adolescents on a mg/m 2 basis.
14. Clinical Studies
The efficacy of COTEMPLA XR-ODT was evaluated in a laboratory classroom study conducted in 87 pediatric patients (Aged 6 to 12 years) with ADHD. Following washout of previous methylphenidate medication, there was an open-label dose-optimization period (4 weeks) with an initial dose of 17.3 mg of COTEMPLA XR-ODT once daily in the morning. The dose could be titrated on a weekly basis from 17.3 mg, to 25.9 mg, to 34.6 mg, and up to 51.8 mg until an optimal dose or the maximum dose of 51.8 mg/day was reached. At the end of this period, subjects remained on their optimized dose for an additional week. Subjects then entered a 1-week randomized, double-blind, parallel group treatment period with the individually optimized dose of COTEMPLA XR-ODT or placebo. At the end of this week, raters evaluated the attention and behavior of the subjects in a laboratory classroom setting, using the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale SKAMP is a validated 13-item teacher-rated scale that assesses manifestations of ADHD in a classroom setting.
The primary efficacy endpoint was the average of the SKAMP-Combined (Attention and Deportment) scores over the test day (not including the baseline score), with assessments conducted at baseline, and 1, 3, 5, 7, 10, 12, and 13 hours post-dosing. The key secondary efficacy endpoints were onset and duration of effect, defined as the first point at which active drug separated from placebo on SKAMP-Combined scores and the last time point at which active drug separated from placebo on SKAMP-Combined scores, respectively.
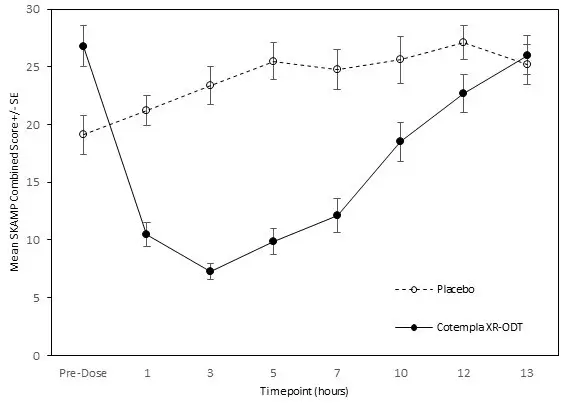
The SKAMP-Combined scores test day average was statistically significantly lower (improved) with COTEMPLA XR-ODT compared to placebo (difference of -11 (95% CI: -13.9, -8.2)) (Table 3).
| Study Number | Treatment Group | Baseline Score at Randomization a (SD) | Pre-dose Score on Classroom Day b (SD) | LS Mean c (SE) | Placebo-subtracted Difference d (95% CI) |
|---|---|---|---|---|---|
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval.
a Visit 7 baseline score (Visit 7 occurred prior to the 1-week randomized, double-blind, parallel group treatment period). b Visit 8 baseline score (Visit 8 occurred at the end of the 1-week randomized, double-blind, parallel group treatment period). c Visit 8 LS mean over hours 1, 3, 5, 7, 10, 12, and 13. d Difference (drug minus placebo) in least-squares means. |
|||||
| Study 1 | Cotempla XR-ODT
(17.3-51.8 mg/day) | 21.1 (9.56) | 26.8 (11.52) | 14.3 (1.07) | -11.0 (-13.9, -8.2) |
| Placebo | 20.4 (9.09) | 19.1 (11.04) | 25.3 (1.16) | -- | |
The SKAMP-Combined scores were also statistically significantly lower (improved) at time points (1, 3, 5, 7, 10, 12 hours) post-dosing with COTEMPLA XR-ODT compared to placebo (Figure 3).
Figure 3: LS Mean SKAMP Combined Score After Treatment with COTEMPLA XR-ODT or Placebo During Classroom Day in Patients with ADHD
*SE = Standard Error
The database was not large enough to assess whether there were differences in effects in age, gender, or race subgroups.
16. How is Cotempla XR-ODT supplied
COTEMPLA XR-ODT Extended Release Orally Disintegrating Tablets are available in three strengths:
- 8.6 mg tablets, round, purple to light purple, mottled, and debossed "T1" on one side of the tablet;
- 17.3 mg tablets, round, purple to light purple, mottled, and debossed "T2" on one side of the tablet;
- 25.9 mg tablets, round, purple to light purple, mottled, and debossed "T3" on one side of the tablet.
They are available as follows:
| NDC 70165-100-30 | 8.6 mg tablets: carton containing 5 blister cards of 6 tablets each, for a total of 30 tablets with a reusable travel case. |
| NDC 70165-200-30 | 17.3 mg tablets: carton containing 5 blister cards of 6 tablets each, for a total of 30 tablets with a reusable travel case. |
| NDC 70165-300-30 | 25.9 mg tablets: carton containing 5 blister cards of 6 tablets each, for a total of 30 tablets with a reusable travel case. |
Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Store COTEMPLA XR-ODT blister packages in the reusable travel case after removal from the carton.
Disposal
Comply with local laws and regulations on drug disposal of CNS stimulants. Dispose of remaining, unused, or expired COTEMPLA XR-ODT by a medicine take-back program or by an authorized collector registered with the Drug Enforcement Administration. If no take-back program or authorized collector is available, mix COTEMPLA XR-ODT with an undesirable, nontoxic substance to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and discard COTEMPLA XR-ODT in the household trash.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Controlled Substance Status/Potential for Abuse and Dependence
Advise patients and their caregivers that COTEMPLA XR-ODT is a federally controlled substance, and it can be abused or lead to dependence [see Drug Abuse and Dependence (9.1, 9.2, and 9.3)] . Instruct patients that they should not give COTEMPLA XR-ODT to anyone else. Advise patients to store COTEMPLA XR-ODT in a safe place, preferably locked, to prevent abuse. Advise patients and their caregivers to comply with laws and regulations on drug disposal. Advise patients and their caregivers to dispose of remaining, unused, or expired COTEMPLA XR-ODT through a medicine take-back program if available [see Warnings and Precautions (5.1), Abuse and Dependence (9.2, 9.3), How Supplied/Storage and Handling (16)] .
Instructions for Taking COTEMPLA XR-ODT
Instruct patients and their caregivers on the following:
- The tablet should remain in the blister pack until the patient is ready to take it.
- The tablet should be taken immediately after opening the blister pack. It should not be stored for future use.
- The patient or caregiver should use dry hands when opening the blister pack.
- The patient or caregiver should remove the tablet by peeling back the foil on the blister pack. The tablet should not be pushed through the foil.
- As soon as the blister is opened, the tablet should be removed and placed on the patient's tongue.
- The whole tablet should be placed on the tongue and allowed to disintegrate without chewing or crushing.
- The tablet will disintegrate in saliva and can be swallowed. No liquid is needed to take the tablet.
Serious Cardiovascular Risks
Advise patients, caregivers, and their family members that there is a potential for serious cardiovascular risks including sudden death, myocardial infarction, and stroke with COTEMPLA XR-ODT. Instruct patients to contact a healthcare provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings and Precautions (5.2)] .
Blood Pressure and Heart Rate Increases
Advise patients and their caregivers that COTEMPLA XR-ODT can elevate blood pressure and heart rate [see Warnings and Precautions (5.3)] .
Psychiatric Risks
Advise patients and their caregivers that COTEMPLA XR-ODT, at recommended doses, can cause psychotic or manic symptoms, even in patients without a prior history or psychotic symptoms or mania [see Warnings and Precautions (5.4)] .
Priapism
Advise patients, caregivers, and family members of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism [see Warnings and Precautions (5.5)] .
Circulation Problems in Fingers and Toes [Peripheral vasculopathy, including Raynaud's phenomenon]
- Instruct patients about the risk of peripheral vasculopathy, including Raynaud's phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking COTEMPLA XR-ODT.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.6)] .
Suppression of Growth
Advise patients, families, and caregivers that COTEMPLA XR-ODT can cause slowing of growth and weight loss [see Warnings and Precautions (5.7)] .
Manufactured for Neos Therapeutics Brands, LLC. Grand Prairie, TX 75050. Made in USA.
For more information, call 1-(888)-319-1789
COTEMPLA XR-ODT is a registered trademark of Neos Therapeutics, Inc.
Copyright © 2014, Neos Therapeutics, Inc.
Item # PIN010299
Rev. 06/2021
Medication Guide
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised June/2021 | ||
| Medication Guide
COTEMPLA XR-ODT (koh-TEM-pluh - oh dee tee) (methylphenidate) extended-release orally disintegrating tablets, CII |
|||
|
What is the most important information I should know about COTEMPLA XR-ODT? |
|||
COTEMPLA XR-ODT can cause serious side effects, including:
|
|||
| What is COTEMPLA XR-ODT? | |||
| COTEMPLA XR-ODT is a central nervous system (CNS) stimulant prescription medicine used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children 6 to 17 years of age. COTEMPLA XR-ODT may help increase attention and decrease impulsiveness and hyperactivity in children 6 to 17 years of age with ADHD. | |||
| It is not known if COTEMPLA XR-ODT is safe and effective in children under 6 years of age. | |||
| COTEMPLA XR-ODT is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep COTEMPLA XR-ODT in a safe place to protect it from theft. Never give your COTEMPLA XR-ODT to anyone else, because it may cause death or harm them. Selling or giving away COTEMPLA XR-ODT may harm others and is against the law. | |||
Do not give COTEMPLA XR-ODT to your child if they are:
|
|||
Before taking COTEMPLA XR-ODT tell your child's healthcare provider about all medical conditions,
including if your child:
|
|||
| Tell your healthcare provider about all of the medicines that your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |||
| COTEMPLA XR-ODT and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted during treatment with COTEMPLA XR-ODT. | |||
Your healthcare provider will decide whether COTEMPLA XR-ODT can be taken with other medicines.
Especially tell your healthcare provider if your child takes:
|
|||
| Know the medicines that your child takes. Keep a list of the medicines with you to show your healthcare provider and pharmacist. Do not start any new medicine during treatment with COTEMPLA XR-ODT without talking to your healthcare provider first. | |||
How should COTEMPLA XR-ODT be taken?
|
|||
| Take COTEMPLA XR-ODT as follows: | |||
|
|||
|
|||
| What should I avoid during treatment with COTEMPLA XR-ODT? | |||
| You should avoid drinking alcohol during treatment with COTEMPLA XR-ODT. | |||
| What are possible side effects of COTEMPLA XR-ODT? | |||
COTEMPLA XR-ODT can cause serious side effects, including:
|
|||
| The most common side effects of methylphenidate products include: | |||
|
| ||
| These are not all the possible side effects of COTEMPLA XR-ODT. | |||
| Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
How should I store COTEMPLA XR-ODT?
|
|||
| Keep COTEMPLA XR-ODT and all medicines out of the reach of children. | |||
| General information about the safe and effective use of COTEMPLA XR-ODT | |||
| Medicines are sometimes prescribed for purposes other than those listed in the Medication Guide. Do not use COTEMPLA XR-ODT for a condition for which it was not prescribed. Do not give COTEMPLA XR-ODT to other people, even if they have the same condition. It may harm them and it is against the law. You can ask your healthcare provider or pharmacist for information about COTEMPLA XR-ODT that was written for healthcare professionals. | |||
| What are the ingredients in COTEMPLA XR-ODT? | |||
| Active Ingredient: Methylphenidate | |||
| Inactive Ingredients: Mannitol, Fructose, Microcrystalline Cellulose, Crospovidone, Methacrylic Acid, Polystyrene Sulfonate, Citric Acid, Colloidal Silicon Dioxide, Grape Flavor, Natural Masking Type Powder, Triethyl Citrate, Magnesium Stearate, Ethylcellulose, Sucralose, Lake Blend Purple, and Polyethylene Glycol | |||
| Manufactured for Neos Therapeutics Brands, LLC, Grand Prairie, TX 75050 | |||
| For more information go to http://www.COTEMPLAXRODT.com or call 1-888-319-1789 | |||
| COTEMPLA XR-ODT is registered in the US Patent and Trademark Office © 2014 Neos Therapeutics, Inc. | |||
PRINCIPAL DISPLAY PANEL - 8.6 mg Tablet Blister Pack Carton
Contains:
NDC 70165-100-30
30 Tablets (5 x 6-count blister cards)
Travel Case
Rx Only
Cotempla XR-ODT™CII
Methylphenidate Extended-Release
Orally Disintegrating Tablets
Do not crush or chew tablets
Each tablet contains 8.6 mg of methylphenidate
(equivalent to that in a 10 mg strength
methylphenidate hydrochloride product)
8.6
mg
NEOS™
Therapeutics
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
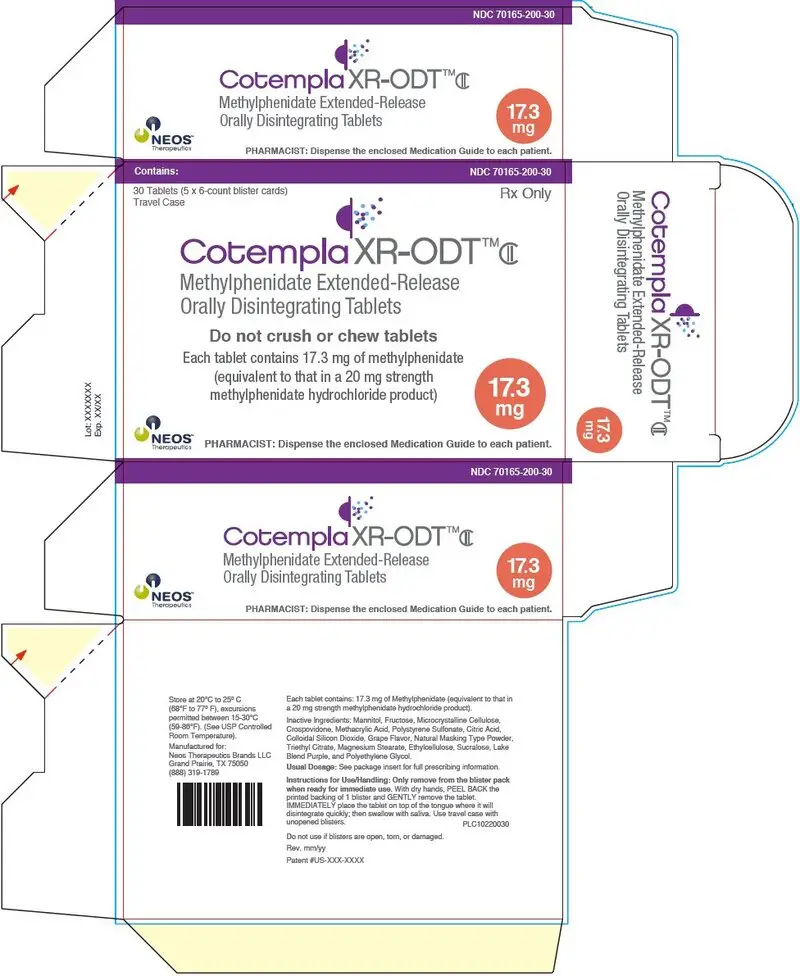
PRINCIPAL DISPLAY PANEL - 17.3 mg Tablet Blister Pack Carton
Contains:
NDC 70165-200-30
30 Tablets (5 x 6-count blister cards)
Travel Case
Rx Only
Cotempla XR-ODT™CII
Methylphenidate Extended-Release
Orally Disintegrating Tablets
Do not crush or chew tablets
Each tablet contains 17.3 mg of methylphenidate
(equivalent to that in a 20 mg strength
methylphenidate hydrochloride product)
17.3
mg
NEOS™
Therapeutics
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
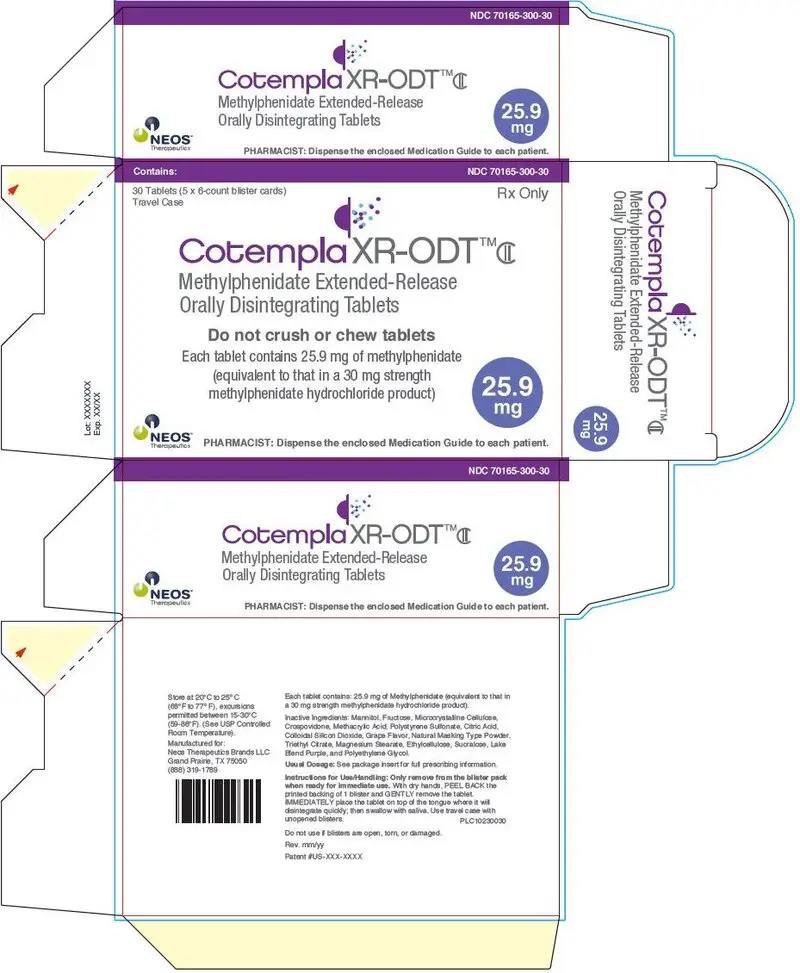
PRINCIPAL DISPLAY PANEL - 25.9 mg Tablet Blister Pack Carton
Contains:
NDC 70165-300-30
30 Tablets (5 x 6-count blister cards)
Travel Case
Rx Only
Cotempla XR-ODT™CII
Methylphenidate Extended-Release
Orally Disintegrating Tablets
Do not crush or chew tablets
Each tablet contains 25.9 mg of methylphenidate
(equivalent to that in a 30 mg strength
methylphenidate hydrochloride product)
25.9
mg
NEOS™
Therapeutics
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
| COTEMPLA XR-ODT
methylphenidate tablet, orally disintegrating |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| COTEMPLA XR-ODT
methylphenidate tablet, orally disintegrating |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| COTEMPLA XR-ODT
methylphenidate tablet, orally disintegrating |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Neos Therapeutics Brands, LLC (080157362) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Neos Therapeutics LP | 836126052 | analysis(70165-100, 70165-200, 70165-300) , label(70165-100, 70165-200, 70165-300) , manufacture(70165-100, 70165-200, 70165-300) , pack(70165-100, 70165-200, 70165-300) | |




