Drug Detail:Creon (Pancrelipase [ pan-kre-lye-pace ])
Drug Class: Digestive enzymes
Highlights of Prescribing Information
CREON (pancrelipase) delayed-release capsules, for oral use
Initial U.S. Approval: 2009
Indications and Usage for Creon
CREON is a combination of porcine-derived lipases, proteases, and amylases indicated for the treatment of exocrine pancreatic insufficiency due to cystic fibrosis, chronic pancreatitis, pancreatectomy, or other conditions. (1)
Creon Dosage and Administration
Administration (2.1)
- CREON is not interchangeable with any other pancrelipase product.
- Do not crush or chew capsules and capsule contents. For infants or patients unable to swallow intact capsules, the contents may be sprinkled on soft acidic food, e.g., applesauce. For complete administration information, see the full prescribing information.
Dosage (2.2)
- CREON is orally administered and dosed by lipase units.
- Dosing should not exceed the recommended maximum dosage set forth by the Cystic Fibrosis Foundation Consensus Conferences Guidelines.
Infants (up to 12 months)
- Prior to each feeding, infants may be given 3,000 lipase units (one capsule) per 120 mL of formula or per breastfeeding. (2.1)
- Do not mix CREON capsule contents directly into formula or breast milk prior to administration. (2.1)
Children Older than 12 Months and Younger than 4 Years
- Begin with 1,000 lipase units/kg of body weight per meal for children less than age 4 years to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day. (2.2)
Children 4 Years and Older and Adults
- Begin with 500 lipase units/kg of body weight per meal for those older than age 4 years to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day. (2.2)
Adults with Exocrine Pancreatic Insufficiency Due to Chronic Pancreatitis or Pancreatectomy
- Individualize dosage based on clinical symptoms, the degree of steatorrhea present and the fat content of the diet. (2.2)
Dosage Forms and Strengths
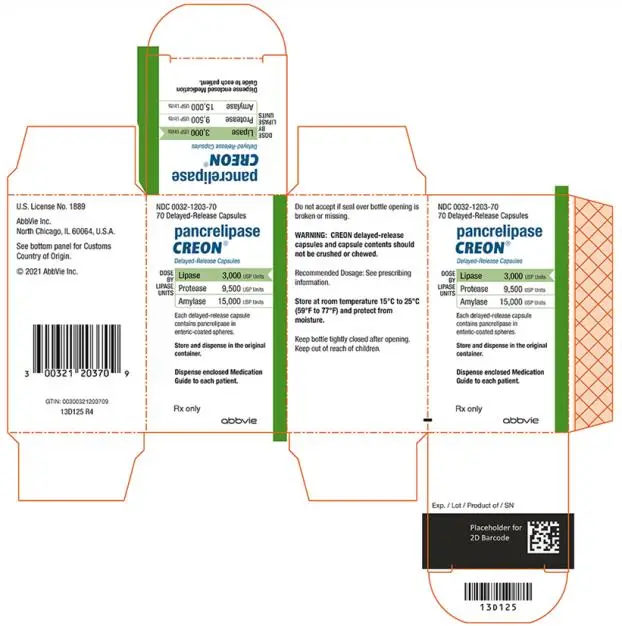
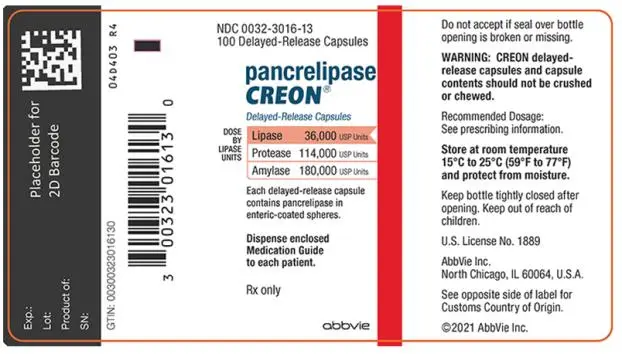
- Delayed-Release Capsules: 3,000 USP units of lipase; 9,500 USP units of protease; and 15,000 USP units of amylase (3)
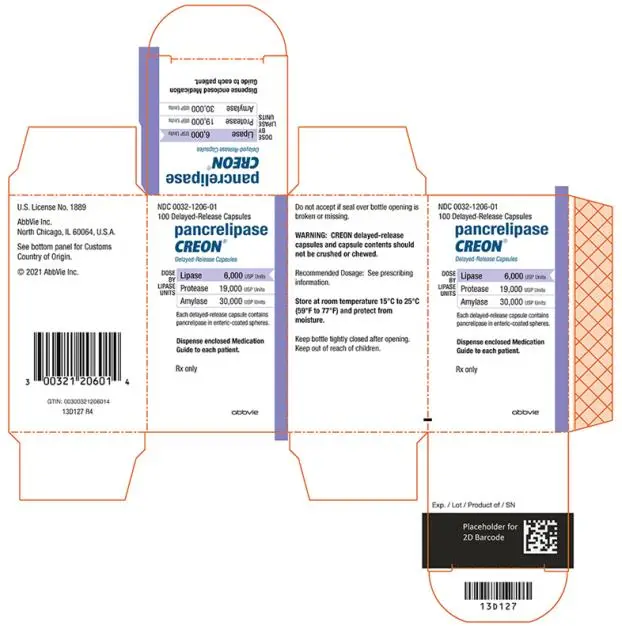
- Delayed-Release Capsules: 6,000 USP units of lipase; 19,000 USP units of protease; and 30,000 USP units of amylase (3)
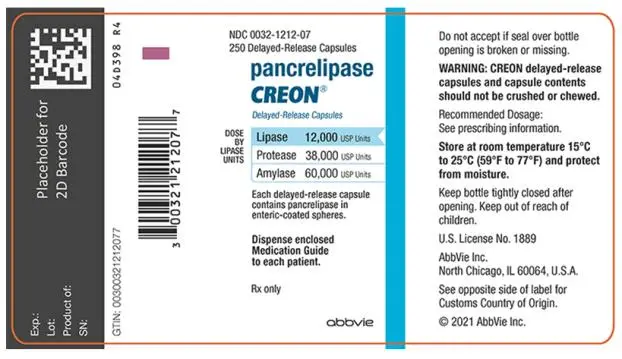
- Delayed-Release Capsules: 12,000 USP units of lipase; 38,000 USP units of protease; and 60,000 USP units of amylase (3)
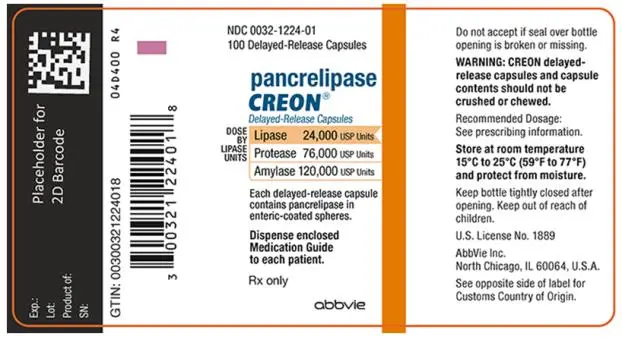
- Delayed-Release Capsules: 24,000 USP units of lipase; 76,000 USP units of protease; and 120,000 USP units of amylase (3)
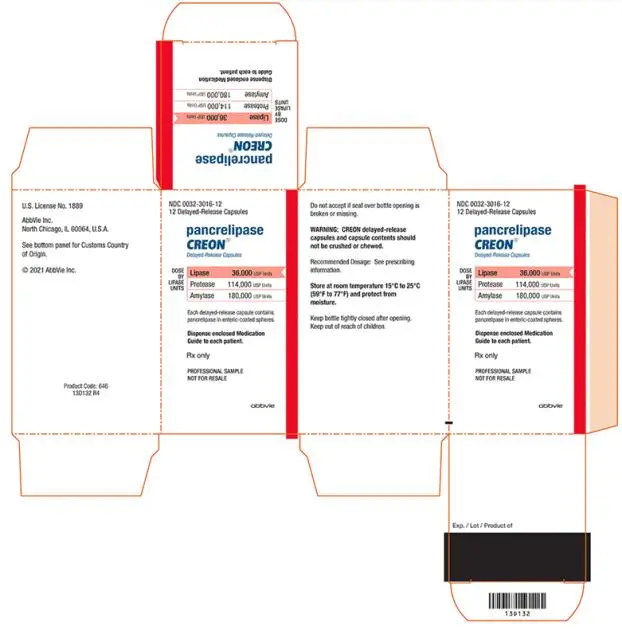
- Delayed-Release Capsules: 36,000 USP units of lipase; 114,000 USP units of protease; and 180,000 USP units of amylase (3)
Contraindications
None (4)
Warnings and Precautions
- Fibrosing colonopathy is associated with high-dose use of pancreatic enzyme replacement in the treatment of cystic fibrosis patients. Exercise caution when doses of CREON exceed 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day). (5.1)
- To avoid irritation of oral mucosa, do not chew CREON or retain in the mouth. (5.2)
- Exercise caution when prescribing CREON to patients with gout, renal impairment, or hyperuricemia. (5.3)
- There is theoretical risk of viral transmission with all pancreatic enzyme products including CREON. (5.4)
- Exercise caution when administering pancrelipase to a patient with a known allergy to proteins of porcine origin. (5.5)
Adverse Reactions/Side Effects
- Adverse reactions occurring in at least 2 cystic fibrosis patients (greater than or equal to 4%) receiving CREON are vomiting, dizziness, and cough. (6.1)
- Adverse reactions that occurred in at least 1 chronic pancreatitis or pancreatectomy patient (greater than or equal to 4%) receiving CREON are hyperglycemia, hypoglycemia, abdominal pain, abnormal feces, flatulence, frequent bowel movements, and nasopharyngitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2022
Related/similar drugs
azithromycin, Zithromax, gentamicin, tobramycin, amikacin, Zenpep, pancrelipaseFull Prescribing Information
1. Indications and Usage for Creon
CREON® is indicated for the treatment of exocrine pancreatic insufficiency due to cystic fibrosis, chronic pancreatitis, pancreatectomy, or other conditions.
2. Creon Dosage and Administration
2.1 Administration
CREON is not interchangeable with other pancrelipase products.
Infants (up to 12 months)
CREON should be administered to infants immediately prior to each feeding, using a dosage of 3,000 lipase units per 120 mL of formula or prior to breastfeeding. Contents of the capsule may be administered directly to the mouth or with a small amount of applesauce. Administration should be followed by breast milk or formula. Contents of the capsule should not be mixed directly into formula or breast milk as this may diminish efficacy. Care should be taken to ensure that CREON is not crushed or chewed or retained in the mouth, to avoid irritation of the oral mucosa.
Children and Adults
CREON should be taken during meals or snacks, with sufficient fluid. CREON capsules and capsule contents should not be crushed or chewed. Capsules should be swallowed whole.
For patients who are unable to swallow intact capsules, the capsules may be carefully opened and the contents added to a small amount of acidic soft food with a pH of 4.5 or less, such as applesauce, at room temperature. The CREON-soft food mixture should be swallowed immediately without crushing or chewing, and followed with water or juice to ensure complete ingestion. Care should be taken to ensure that no drug is retained in the mouth.
2.2 Dosage
CREON is orally administered and dosed by lipase units. Therapy should be initiated at the lowest recommended dose and gradually increased. The dosage of CREON should be individualized based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet as described in the Limitations on Dosing below [see also Warnings and Precautions (5.1)].
Dosage recommendations for pancreatic enzyme replacement therapy were published following the Cystic Fibrosis Foundation Consensus Conferences.1, 2, 3 CREON should be administered in a manner consistent with the recommendations of the Cystic Fibrosis Foundation Consensus Conferences (also known as Conferences) provided in the following paragraphs, except for infants. Although the Conferences recommend doses of 2,000 to 4,000 lipase units in infants up to 12 months, CREON is available in a 3,000 lipase unit capsule. Therefore, the recommended dose of CREON in infants up to 12 months is 3,000 lipase units per 120 mL of formula or per breastfeeding. Patients may be dosed on a fat ingestion-based or actual body weight-based dosing scheme.
Additional recommendations for pancreatic enzyme therapy in patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatectomy are based on a clinical trial conducted in these populations.
Infants (up to 12 months)
CREON is available in the strength of 3,000 USP units of lipase thus infants may be given 3,000 lipase units (one capsule) per 120 mL of formula or per breastfeeding. Do not mix CREON capsule contents directly into formula or breast milk prior to administration [see Administration (2.1)].
Children Older than 12 Months and Younger than 4 Years
Enzyme dosing should begin with 1,000 lipase units/kg of body weight per meal for children less than age 4 years to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day.
Children 4 Years and Older and Adults
Enzyme dosing should begin with 500 lipase units/kg of body weight per meal for those older than age 4 years to a maximum of 2,500 lipase units/kg of body weight per meal (or less than or equal to 10,000 lipase units/kg of body weight per day), or less than 4,000 lipase units/g fat ingested per day.
Usually, half of the prescribed CREON dose for an individualized full meal should be given with each snack. The total daily dose should reflect approximately three meals plus two or three snacks per day.
Enzyme doses expressed as lipase units/kg of body weight per meal should be decreased in older patients because they weigh more but tend to ingest less fat per kilogram of body weight.
Adults with Exocrine Pancreatic Insufficiency Due to Chronic Pancreatitis or Pancreatectomy
The initial starting dose and increases in the dose per meal should be individualized based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet.
In one clinical trial, patients received CREON at a dose of 72,000 lipase units per meal while consuming at least 100 g of fat per day [see Clinical Studies (14.2)]. Lower starting doses recommended in the literature are consistent with the 500 lipase units/kg of body weight per meal lowest starting dose recommended for adults in the Cystic Fibrosis Foundation Consensus Conferences Guidelines.1, 2, 3, 4 Usually, half of the prescribed CREON dose for an individualized full meal should be given with each snack.
Limitations on Dosing
Dosing should not exceed the recommended maximum dosage set forth by the Cystic Fibrosis Foundation Consensus Conferences Guidelines.1, 2, 3 If symptoms and signs of steatorrhea persist, the dosage may be increased by the healthcare professional. Patients should be instructed not to increase the dosage on their own. There is great inter-individual variation in response to enzymes; thus, a range of doses is recommended. Changes in dosage may require an adjustment period of several days. If doses are to exceed 2,500 lipase units/kg of body weight per meal, further investigation is warranted. Doses greater than 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day) should be used with caution and only if they are documented to be effective by 3-day fecal fat measures that indicate a significantly improved coefficient of fat absorption. Doses greater than 6,000 lipase units/kg of body weight per meal have been associated with colonic stricture, indicative of fibrosing colonopathy, in children less than 12 years of age [see Warnings and Precautions (5.1)]. Patients currently receiving higher doses than 6,000 lipase units/kg of body weight per meal should be examined and the dosage either immediately decreased or titrated downward to a lower range.
3. Dosage Forms and Strengths
Delayed-release capsules are available in the following strengths:
- 3,000 USP units of lipase; 9,500 USP units of protease; and 15,000 USP units of amylase in a two-piece hypromellose capsule with a white opaque cap imprinted with “CREON 1203” and a white opaque body.
- 6,000 USP units of lipase; 19,000 USP units of protease; and 30,000 USP units of amylase in a two-piece gelatin capsule with an orange opaque cap imprinted with “CREON 1206” and a blue opaque body.
- 12,000 USP units of lipase; 38,000 USP units of protease; and 60,000 USP units of amylase in a two-piece gelatin capsule with a brown opaque cap imprinted with “CREON 1212” and a colorless transparent body.
- 24,000 USP units of lipase; 76,000 USP units of protease; and 120,000 USP units of amylase in a two-piece gelatin capsule with an orange opaque cap imprinted with “CREON 1224” and a colorless transparent body.
- 36,000 USP units of lipase; 114,000 USP units of protease; and 180,000 USP units of amylase in a two-piece gelatin capsule with a blue opaque cap imprinted with “CREON 1236” and a colorless transparent body.
5. Warnings and Precautions
5.1 Fibrosing Colonopathy
Fibrosing colonopathy has been reported following treatment with different pancreatic enzyme products. 5, 6 Fibrosing colonopathy is a rare, serious adverse reaction initially described in association with high-dose pancreatic enzyme use, usually over a prolonged period of time and most commonly reported in pediatric patients with cystic fibrosis. The underlying mechanism of fibrosing colonopathy remains unknown. Doses of pancreatic enzyme products exceeding 6,000 lipase units/kg of body weight per meal have been associated with colonic stricture in children less than 12 years of age.1 Patients with fibrosing colonopathy should be closely monitored because some patients may be at risk of progressing to stricture formation. It is uncertain whether regression of fibrosing colonopathy occurs.1 It is generally recommended, unless clinically indicated, that enzyme doses should be less than 2,500 lipase units/kg of body weight per meal (or less than 10,000 lipase units/kg of body weight per day) or less than 4,000 lipase units/g fat ingested per day [see Dosage and Administration (2.1)].
Doses greater than 2,500 lipase units/kg of body weight per meal (or greater than 10,000 lipase units/kg of body weight per day) should be used with caution and only if they are documented to be effective by 3-day fecal fat measures that indicate a significantly improved coefficient of fat absorption. Patients receiving higher doses than 6,000 lipase units/kg of body weight per meal should be examined and the dosage either immediately decreased or titrated downward to a lower range.
5.2 Potential for Irritation to Oral Mucosa
Care should be taken to ensure that no drug is retained in the mouth. CREON should not be crushed or chewed or mixed in foods having a pH greater than 4.5. These actions can disrupt the protective enteric coating resulting in early release of enzymes, irritation of oral mucosa, and/or loss of enzyme activity [see Dosage and Administration (2.2) and Patient Counseling Information (17.1)]. For patients who are unable to swallow intact capsules, the capsules may be carefully opened and the contents added to a small amount of acidic soft food with a pH of 4.5 or less, such as applesauce, at room temperature. The CREON-soft food mixture should be swallowed immediately and followed with water or juice to ensure complete ingestion.
5.3 Potential for Risk of Hyperuricemia
Caution should be exercised when prescribing CREON to patients with gout, renal impairment, or hyperuricemia. Porcine-derived pancreatic enzyme products contain purines that may increase blood uric acid levels.
5.4 Potential Viral Exposure from the Product Source
CREON is sourced from pancreatic tissue from swine used for food consumption. Although the risk that CREON will transmit an infectious agent to humans has been reduced by testing for certain viruses during manufacturing and by inactivating certain viruses during manufacturing, there is a theoretical risk for transmission of viral disease, including diseases caused by novel or unidentified viruses. Thus, the presence of porcine viruses that might infect humans cannot be definitely excluded. However, no cases of transmission of an infectious illness associated with the use of porcine pancreatic extracts have been reported.
5.5 Allergic Reactions
Caution should be exercised when administering pancrelipase to a patient with a known allergy to proteins of porcine origin. Rarely, severe allergic reactions including anaphylaxis, asthma, hives, and pruritus, have been reported with other pancreatic enzyme products with different formulations of the same active ingredient (pancrelipase). The risks and benefits of continued CREON treatment in patients with severe allergy should be taken into consideration with the overall clinical needs of the patient.
6. Adverse Reactions/Side Effects
The most serious adverse reactions reported with different pancreatic enzyme products of the same active ingredient (pancrelipase) that are described elsewhere in the label include fibrosing colonopathy, hyperuricemia and allergic reactions [see Warnings and Precautions (5)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The short-term safety of CREON was assessed in clinical trials conducted in 121 patients with exocrine pancreatic insufficiency (EPI): 67 patients with EPI due to cystic fibrosis (CF) and 25 patients with EPI due to chronic pancreatitis or pancreatectomy were treated with CREON.
Cystic Fibrosis
Studies 1 and 2 were randomized, double-blind, placebo-controlled, crossover studies of 49 patients, ages 7 to 43 years, with EPI due to CF. Study 1 included 32 patients ages 12 to 43 years and Study 2 included 17 patients ages 7 to 11 years. In these studies, patients were randomized to receive CREON at a dose of 4,000 lipase units/g fat ingested per day or matching placebo for 5 to 6 days of treatment, followed by crossover to the alternate treatment for an additional 5 to 6 days. The mean exposure to CREON during these studies was 5 days.
In Study 1, one patient experienced duodenitis and gastritis of moderate severity 16 days after completing treatment with CREON. Transient neutropenia without clinical sequelae was observed as an abnormal laboratory finding in one patient receiving CREON and a macrolide antibiotic.
In Study 2, adverse reactions that occurred in at least 2 patients (greater than or equal to 12%) treated with CREON were vomiting and headache. Vomiting occurred in 2 patients treated with CREON and did not occur in patients treated with placebo; headache occurred in 2 patients treated with CREON and did not occur in patients treated with placebo.
The most common adverse reactions (greater than or equal to 4%) in Studies 1 and 2 were vomiting, dizziness, and cough. Table 1 enumerates adverse reactions that occurred in at least 2 patients (greater than or equal to 4%) treated with CREON at a higher rate than with placebo in Studies 1 and 2.
| Adverse Reaction | CREON Capsules
n = 49 (%) | Placebo
n = 47 (%) |
| Vomiting | 3 (6) | 1 (2) |
| Dizziness | 2 (4) | 1 (2) |
| Cough | 2 (4) | 0 |
An additional open-label, single-arm study assessed the short-term safety and tolerability of CREON in 18 infants and children, ages 4 months to 6 years, with EPI due to cystic fibrosis. Patients received their usual pancreatic enzyme replacement therapy (mean dose of 7,000 lipase units/kg/day for a mean duration of 18.2 days) followed by CREON (mean dose of 7,500 lipase units/kg/day for a mean duration of 12.6 days). There were no serious adverse reactions. Adverse reactions that occurred in patients during treatment with CREON were vomiting, irritability, and decreased appetite, each occurring in 6% of patients.
Chronic Pancreatitis or Pancreatectomy
A randomized, double-blind, placebo-controlled, parallel group study was conducted in 54 adult patients, ages 32 to 75 years, with EPI due to chronic pancreatitis or pancreatectomy. Patients received single-blind placebo treatment during a 5-day run-in period followed by an intervening period of up to 16 days of investigator-directed treatment with no restrictions on pancreatic enzyme replacement therapy. Patients were then randomized to receive CREON or matching placebo for 7 days. The CREON dose was 72,000 lipase units per main meal (3 main meals) and 36,000 lipase units per snack (2 snacks). The mean exposure to CREON during this study was 6.8 days in the 25 patients that received CREON.
The most common adverse reactions reported during the study were related to glycemic control and were reported more commonly during CREON treatment than during placebo treatment.
Table 2 enumerates adverse reactions that occurred in at least 1 patient (greater than or equal to 4%) treated with CREON at a higher rate than with placebo.
| Adverse Reaction | CREON Capsules
n = 25 (%) | Placebo
n = 29 (%) |
| Hyperglycemia | 2 (8) | 2 (7) |
| Hypoglycemia | 1 (4) | 1 (3) |
| Abdominal Pain | 1 (4) | 1 (3) |
| Abnormal Feces | 1 (4) | 0 |
| Flatulence | 1 (4) | 0 |
| Frequent Bowel Movements | 1 (4) | 0 |
| Nasopharyngitis | 1 (4) | 0 |
14. Clinical Studies
The short-term efficacy of CREON was evaluated in three studies conducted in 103 patients with exocrine pancreatic insufficiency (EPI). Two studies were conducted in 49 patients with EPI due to cystic fibrosis (CF); one study was conducted in 54 patients with EPI due to chronic pancreatitis or pancreatectomy.
14.2 Chronic Pancreatitis or Pancreatectomy
A randomized, double-blind, placebo-controlled, parallel group study was conducted in 54 adult patients, ages 32 to 75 years, with EPI due to chronic pancreatitis or pancreatectomy. The final analysis population was limited to 52 patients; 2 patients were excluded due to protocol violations. Ten patients had a history of pancreatectomy (7 were treated with CREON). In this study, patients received placebo for 5 days (run-in period), followed by pancreatic enzyme replacement therapy as directed by the investigator for 16 days; this was followed by randomization to CREON or matching placebo for 7 days of treatment (double-blind period). Only patients with CFA less than 80% in the run-in period were randomized to the double-blind period. The dose of CREON during the double-blind period was 72,000 lipase units per main meal (3 main meals) and 36,000 lipase units per snack (2 snacks). All patients consumed a high-fat diet (greater than or equal to 100 grams of fat per day) during the treatment period.
The CFA was determined by a 72-hour stool collection during the run-in and double-blind treatment periods, when both fat excretion and fat ingestion were measured. The mean change in CFA from the run-in period to the end of the double-blind period in the CREON and Placebo groups is shown in Table 3.
| CREON
n = 24 | Placebo
n = 28 |
|
| CFA [%] | ||
| Run-in Period (Mean, SD) | 54 (19) | 57 (21) |
| End of Double-Blind Period (Mean, SD) | 86 (6) | 66 (20) |
| Change in CFA * [%] | ||
| Run-in Period to End of Double-Blind Period (Mean, SD) | 32 (18) | 9 (13) |
| Treatment Difference (95% CI) | 21 (14, 28) | |
| *p<0.0001 | ||
Subgroup analyses of the CFA results showed that mean change in CFA was greater in patients with lower run-in period CFA values than in patients with higher run-in period CFA values. Only 1 of the patients with a history of total pancreatectomy was treated with CREON in the study. That patient had a CFA of 26% during the run-in period and a CFA of 73% at the end of the double-blind period. The remaining 6 patients with a history of partial pancreatectomy treated with CREON on the study had a mean CFA of 42% during the run-in period and a mean CFA of 84% at the end of the double-blind period.
17. Patient Counseling Information
See FDA-approved patient labeling (Medication Guide).
17.1 Dosing and Administration
- Instruct patients and caregivers that CREON should only be taken as directed by their healthcare professional. Patients should be advised that the total daily dose should not exceed 10,000 lipase units/kg body weight/day unless clinically indicated. This needs to be especially emphasized for patients eating multiple snacks and meals per day. Patients should be informed that if a dose is missed, the next dose should be taken with the next meal or snack as directed. Doses should not be doubled [see Dosage and Administration (2)].
- Instruct patients and caregivers that CREON should always be taken with food. Patients should be advised that CREON delayed-release capsules and the capsule contents must not be crushed or chewed as doing so could cause early release of enzymes and/or loss of enzymatic activity. Patients should swallow the intact capsules with adequate amounts of liquid at mealtimes. If necessary, the capsule contents can also be sprinkled on soft acidic foods [see Dosage and Administration (2)].
Medication Guide
| MEDICATION GUIDE
CREON® (krē ′ŏn) (pancrelipase) delayed-release capsules, for oral use |
| Read this Medication Guide before you start taking CREON and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment. |
| What is the most important information I should know about CREON?
CREON may increase your chance of having a rare bowel disorder called fibrosing colonopathy. This condition is serious and may require surgery. The risk of having this condition may be reduced by following the dosing instructions that your doctor gave you. Call your doctor right away if you have any unusual or severe:
|
| What is CREON?
CREON is a prescription medicine used to treat people who cannot digest food normally because their pancreas does not make enough enzymes due to cystic fibrosis, swelling of the pancreas that lasts a long time (chronic pancreatitis), removal of some or all of the pancreas (pancreatectomy), or other conditions. CREON may help your body use fats, proteins, and sugars from food. CREON contains a mixture of digestive enzymes including lipases, proteases, and amylases from pig pancreas. What should I tell my doctor before taking CREON? Before taking CREON, tell your doctor about all your medical conditions, including if you:
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. |
How should I take CREON?
|
| What are the possible side effects of CREON?
CREON may cause serious side effects, including:
The most common side effects of CREON include:
CREON and other pancreatic enzyme products are made from the pancreas of pigs, the same pigs people eat as pork. These pigs may carry viruses. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs. Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the side effects of CREON. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088. You may also report side effects to AbbVie Inc. at 1-800-633-9110. |
How should I store CREON?
|
| General information about CREON
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use CREON for a condition for which it was not prescribed. Do not give CREON to other people to take, even if they have the same symptoms you have. It may harm them. This Medication Guide summarizes the most important information about CREON. If you would like more information, talk to your doctor. You can ask your doctor or pharmacist for information about CREON that is written for healthcare professionals. For more information, go to www.creon-us.com or call toll-free [1-800-633-9110]. |
| What are the ingredients in CREON?
Active Ingredient: lipase, protease, amylase Inactive Ingredients: cetyl alcohol, dimethicone, hypromellose phthalate, polyethylene glycol, and triethyl citrate. The shells of the CREON 6,000 USP units of lipase, 12,000 USP units of lipase, and 24,000 USP units of lipase strengths contain: gelatin, red iron oxide, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide. In addition: The shells for the CREON 3,000 USP units of lipase strength capsules contain titanium dioxide and hypromellose. The shells of the CREON 6,000 USP units of lipase strength capsules contain FD&C Blue No. 2. The shells of the CREON 12,000 USP units of lipase strength capsules contain black iron oxide. The shells of the CREON 36,000 USP units of lipase strength capsules contain gelatin, titanium dioxide, sodium lauryl sulfate and FD&C Blue No. 2. This Medication Guide has been approved by the U.S. Food and Drug Administration. AbbVie Inc. North Chicago, IL 60064, U.S.A. US License Number 1889 © 2009-2022 AbbVie Inc. Revised: June, 2022 20072271 |
| CREON
pancrelipase capsule, delayed release pellets |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| CREON
pancrelipase capsule, delayed release pellets |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| CREON
pancrelipase capsule, delayed release pellets |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CREON
pancrelipase capsule, delayed release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CREON
pancrelipase capsule, delayed release pellets |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |