Drug Class: Mydriatics
Cyclomydril Description
CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is a mydriatic prepared as a sterile topical ophthalmic solution. The active ingredients are represented by the chemical structures:
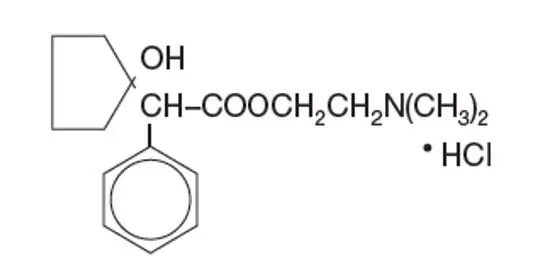
Established Name:
Cyclopentolate Hydrochloride
Chemical Name:
2-(Dimethylamino)ethyl 1 - hydroxy-α-phenylcyclopentaneacetate hydrochloride)
Molecular Formula:
Molecular Weight:
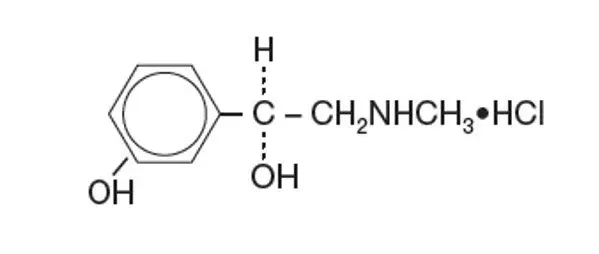
Established Name:
Phenylephrine Hydrochloride
Chemical Name:
3-hydroxy-α[(methylamino)-methyl]-, Benzenemethanol, hydrochloride (R)-.
Molecular Formula:
Molecular Weight:
Each mL of CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) contains: Active: cyclopentolate hydrochloride 0.2%, phenylephrine hydrochloride 1%. Preservative: benzalkonium chloride 0.01%. Inactives: edetate disodium, boric acid, hydrochloric acid and /or sodium carbonate (to adjust pH), purified water.
Cyclomydril - Clinical Pharmacology
Cyclopentolate hydrochloride is an anticholinergic drug and phenylephrine hydrochloride is an adrenergic drug. This combination induces mydriasis that is greater than that of either drug alone at its respective concentration. The concentrations of cyclopentolate hydrochloride and phenylephrine hydrochloride have been selected to induce mydriasis with little accompanying cycloplegia. Heavily pigmented irides may require more doses than lightly pigmented irides.
Contraindications
Do not use in patients with untreated narrow-angle glaucoma or with untreated anatomically narrow angles or where there is hypersensitivity to any component of this preparation.
Warnings
FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION. The use of this combination may have an adverse effect on individuals suffering from cardiovascular disease, hypertension, and hyperthyroidism, and it may cause CNS disturbances. Infants are especially prone to CNS and cardiopulmonary side effects from cyclopentolate. Observe infants closely for at least 30 minutes.
Mydriatics may produce a transient elevation of intraocular pressure.
Precautions
General
The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation to reduce excessive systemic absorption. Caution should be observed when considering use of this medication in the presence of Down's syndrome and in those predisposed to angle-closure glaucoma. The effect of long-term use of this preparation has not been established, therefore, it should be restricted to short-term use.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the solution. Patient should be advised not to drive or engage in hazardous activities while pupils are dilated. Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration. Feeding intolerance may follow ophthalmic use of this product in infants. It is recommended that feeding be withheld for four (4) hours after examination.
Drug Interactions
Cyclopentolate may interfere with the ocular anti-hypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no long-term studies done using cyclopentolate hydrochloride and/or phenylephrine hydrochloride in animals to evaluate carcinogenic potential.
Pregnancy
Animal reproduction studies have not been conducted with cyclopentolate hydrochloride and/or phenylephrine hydrochloride. It is also not known whether cyclopentolate hydrochloride and/or phenylephrine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether these drugs are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is administered to a nursing woman.
Pediatric Use
Use of cyclopentolate has been associated with psychotic reactions and behavioral disturbances in pediatric patients. Increased susceptibility to cyclopentolate has been reported in infants, young children, and in children with spastic paralysis or brain damage. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place, and failure to recognize people. Feeding intolerance may follow ophthalmic use of this product in infants. It is recommended that feeding be withheld for 4 hours after examination. Observe infants closely for at least 30 minutes (see PRECAUTIONS).
Adverse Reactions/Side Effects
Ocular
The following ocular adverse experiences have been associated with the use of CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution): increased intraocular pressure, burning/irritation upon instillation, photophobia, blurred vision and superficial punctate keratitis.
Nonocular
Use of cyclopentolate hydrochloride has been associated with psychotic reactions and behavioral disturbances in children. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place, and failure to recognize people. This drug produces reactions similar to those of other adrenergic and anticholinergic drugs; however, the central nervous system manifestations as noted above are most common. Other manifestations of adrenergic and anticholinergic topical ophthalmic drugs include tachycardia, hyperpyrexia, hypertension, vasodilation, urinary retention, diminished gastrointestinal motility, and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Severe manifestations of toxicity include coma, medullary paralysis and death.
Overdosage
Excessive dosage may produce behavioral disturbances, tachycardia, hyperpyrexia, hypertension, elevated intraocular pressure, vasodilation, urinary retention, diminished gastrointestinal motility and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Patients exhibiting signs of overdosage should receive supportive care and monitoring.
Cyclomydril Dosage and Administration
Instill one drop in each eye every five to ten minutes. To minimize systemic absorption, apply pressure over the nasolacrimal sac for two to three minutes following instillation. Observe infants closely for at least 30 minutes.
How is Cyclomydril supplied
CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is supplied as a sterile solution in 2 mL and 5 mL, in plastic DROP-TAINER® dispensers.
2 mL NDC 0065-0359-02
5 mL NDC 0065-0359-05
Storage: Store at 8°C to 25°C (46°F - 77°F)
Distributed by:
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134-2099
©2004, 2013, 2018, 2021 Alcon Inc.
T2018-97
Revised: June 2018
PRINCIPAL DISPLAY PANEL
NDC 0065-0359-05
Alcon
Cyclomydril®
(cyclopentolate
hydrochloride,
phenylephrine
hydrochloride
ophthalmic
solution)
5 mL Sterile
Rx Only
Sterile Ophthalmic Solution
USUAL DOSAGE: Instill one drop
in each eye every five to ten
minutes. Read enclosed insert.
FOR TOPICAL OPHTHALMIC USE
ONLY
WARNING: Do not touch
dropper tip to any surface as
this may contaminate the
solution.
INGREDIENTS: Each mL
contains: Active:
cyclopentolate hydrochloride
0.2%, phenylephrine
hydrochloride 1%.
Preservative: benzalkonium
chloride 0.01%. Inactives:
edetate disodium, boric acid,
hydrochloric acid and/or
sodium carbonate (to adjust
pH), purified water.
STORAGE: Store at 8°-25° C
(46° to 77° F).
Alcon
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
SN:
LOT:
EXP.:
GTIN: 00300650359054
300048632-0821
NDC 0065-0359-05
Alcon
Cyclomydril®
(cyclopentolate hydrochloride, phenylephrine hydrochloride ophthalmic solution)
5 mL Sterile
Rx Only
Sterile Ophthalmic Solution
USUAL DOSAGE: Instill one drop in each eye every five to ten minutes. Read enclosed insert.
FOR TOPICAL OPHTHALMIC USE ONLY
WARNING: Do not touch dropper tip to any surface as this may contaminate the solution.
INGREDIENTS: Each mL contains: Active: cyclopentolate hydrochloride 0.2%, phenylephrine hydrochloride 1%. Preservative: benzalkonium chloride 0.01%. Inactives: edetate disodium, boric acid, hydrochloric acid and/or sodium carbonate (to adjust pH), purified water.
STORAGE: Store at 8°-25° C (46° to 77° F).
©2004, 2010, 2013 Novartis
9014758 US
Alcon®
a Novartis company
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
LOT:
EXP.:

NDC 0065-0359-05
Alcon
Cyclomydril®
(cyclopentolate
hydrochloride,
phenylephrine
hydrochloride
ophthalmic
solution)
Sterile 5 mL
Rx Only
INGREDIENTS: Each mL contains: Actives: cyclopentolate
hydrochloride 0.2%, phenylephrine hydrochloride 1%.
Preservative: benzalkonium chloride 0.01%. Inactives:
edetate disodium, boric acid, hydrochloric acid and/or
sodium carbonate (to adjust pH), purified water.
WARNING: Do not touch dropper tip to any surface as this
may contaminate the solution.
USUAL DOSAGE: Instill one drop in each eye every five to
ten minutes. Read enclosed insert.
STORAGE: Store at 8°-25° C (46° to 77° F).
Printed in USA
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
LOT:
EXP.:
300048631-0821

NDC 0065-0359-05
Alcon
Cyclomydril®
(cyclopentolate hydrochloride, phenylephrine hydrochloride ophthalmic solution)
Sterile 5 mL
Rx Only
INGREDIENTS: Each mL contains: Actives: cyclopentolate hydrochloride 0.2%, phenylephrine hydrochloride 1%. Preservative: benzalkonium chloride 0.01%. Inactives: edetate disodium, boric acid, hydrochloric acid and/or sodium carbonate (to adjust pH), purified water.
WARNING: Do not touch dropper tip to any surface, as this may contaminate the solution.
USUAL DOSAGE: Instill one drop in each eye every five to ten minutes. Read enclosed insert.
STORAGE: Store at 8°-25°C (46°-77°F).
Printed in USA
©2004, 2013 Novartis
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
LOT:
EXP.:
H14882 US
| CYCLOMYDRIL
cyclopentolate hydrochloride and phenylephrine hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcon Research LLC | 007672236 | manufacture(0065-0359) | |




