Drug Detail:Cysview (Hexaminolevulinate hydrochloride)
Drug Class: Malignancy photosensitizers
Highlights of Prescribing Information
Cysview (hexaminolevulinate hydrochloride), for Intravesical Solution For bladder instillation only
Initial U.S. Approval: 2010
Indications and Usage for Cysview
Cysview is an optical imaging agent indicated for use in the cystoscopic detection of carcinoma of the bladder, including carcinoma in situ (CIS), among patients suspected or known to have lesion(s) on the basis of a prior cystoscopy, or in patients undergoing surveillance cystoscopy for carcinoma of the bladder. Cysview is used with the Karl Storz D-Light C Photodynamic Diagnostic (PDD) system to perform Blue Light Cystoscopy (BLC ®) as an adjunct to the white light cystoscopy.
Important Limitations of Use:
- Not a replacement for random bladder biopsies or other procedures used in the detection of bladder cancer. ( 1.1, 5.2)
Cysview Dosage and Administration
Training in blue light cystoscopy with the Karl Storz D-Light C PDD system is essential prior to the use of Cysview. ( 2.5)
- Reconstitute Cysview powder with the supplied 50 mL DILUENT under aseptic conditions. ( 2.2)
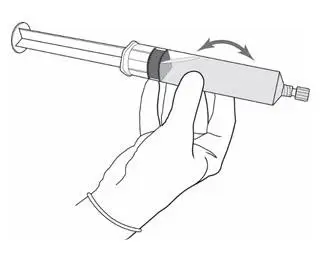
- Use solution of Cysview shortly after reconstitution. If unable to use, the solution may be stored for up to 2 hours in a refrigerator at 2°-8°C (36°-46°F) in the labeled syringe. Discard after 2 hours. ( 2.2, 16)
- Instill 50 mL of reconstituted solution of Cysview into the emptied bladder via an intravesical catheter. Retain in the bladder for 1 hour before evacuating and performing cystoscopic examination. ( 2.3, 2.5)
- First perform a complete cystoscopic examination of the entire bladder under white light and then repeat the examination of the entire bladder under blue light. Record and document information about location and appearance of suspicious lesions and areas seen under both white and blue light. ( 2.5)
Dosage Forms and Strengths
Cysview (hexaminolevulinate hydrochloride) is supplied as a kit. The kit may be supplied as two options; with or without a vial adapter:
- One 10 mL glass vial containing 100 mg powder of Cysview. (hexaminolevulinate hydrochloride) for Intravesical Solution.
- One plastic prefilled syringe containing 50 mL DILUENT for Cysview.
- One Luer Lock catheter adapter.
- One vial adapter for use during reconstitution (in the kit containing the vial adapter). ( 16)
Once reconstituted, the solution contains 2 mg/mL (8 mmol/L) of hexaminolevulinate hydrochloride.
Contraindications
Do not use Cysview in patients with:
- porphyria,
- gross hematuria,
- known hypersensitivity to hexaminolevulinate or aminolevulinate derivatives. ( 4)
Warnings and Precautions
- Anaphylaxis: have trained personnel and therapies available. ( 5.1).
- Failed Detection: Cysview may not detect all malignant lesions. Always perform white light cystoscopy followed by blue light cystoscopy. Do not biopsy with blue light only. ( 5.2)
- False fluorescence may occur due to inflammation, cystoscopic trauma, scar tissue, previous bladder biopsy, recent BCG therapy or chemotherapy. ( 5.3)
Adverse Reactions/Side Effects
The most common adverse reaction reported in patients who received Cysview was bladder spasm, occurring in 2% of patients, followed by dysuria, hematuria and bladder pain. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Photocure Inc. at 1-855-297-8439 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pediatric Use: Safety and effectiveness in pediatric patients have not been established. ( 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
Related/similar drugs
Lexiscan, glucagon, mannitol, arginine, CeretecFull Prescribing Information
1. Indications and Usage for Cysview
Cysview is indicated for use in the cystoscopic detection of carcinoma of the bladder, including carcinoma in situ (CIS), among patients suspected or known to have lesion(s) on the basis of a prior cystoscopy, or in patients undergoing surveillance cystoscopy for carcinoma of the bladder. Cysview is used with the Karl Storz D-Light C Photodynamic Diagnostic (PDD) system to perform Blue Light Cystoscopy (BLC ®) as an adjunct to the white light cystoscopy.
2. Cysview Dosage and Administration
2.1 Recommended Dose
The recommended dose for adults is 50 mL of reconstituted solution of Cysview [ see Dosage and Administration (2.2)], instilled into the bladder via a urinary catheter [ see Dosage and Administration (2.3)].
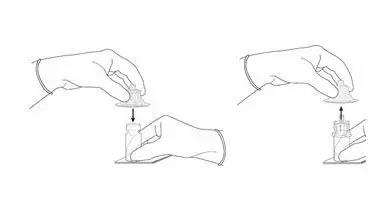
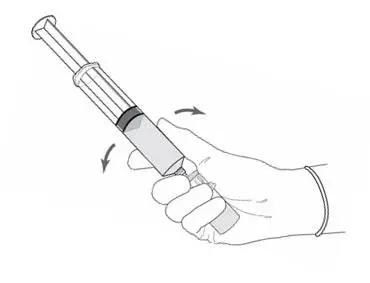
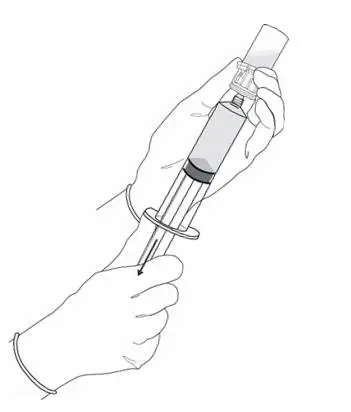
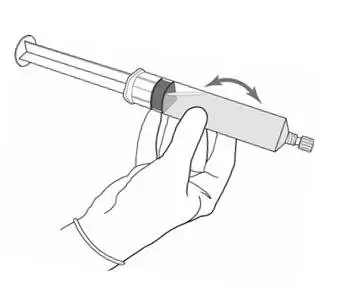
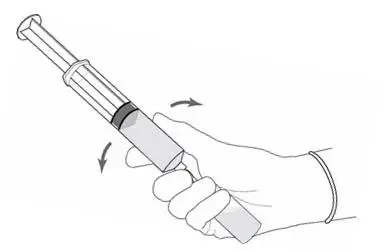
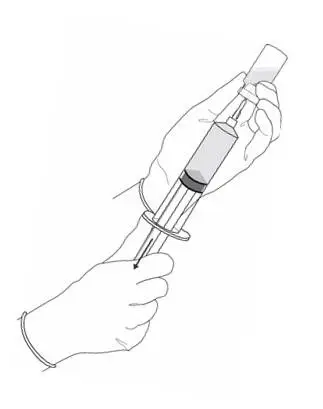
2.2 Reconstitution of Cysview
Cysview is supplied as a kit containing: a clear glass vial labeled as Cysview (hexaminolevulinate HCl) for Intravesical Solution containing 100 mg hexaminolevulinate hydrochloride as a powder, a prefilled syringe labeled as DILUENT for Cysview containing 50 mL of the diluent, and a catheter adapter. The kit may be supplied as two options; with or without a vial adapter for use during reconstitution.
Perform all steps under aseptic conditions. Wear gloves during the reconstitution procedure; skin exposure to hexaminolevulinate hydrochloride may increase the risk for sensitization to the drug.
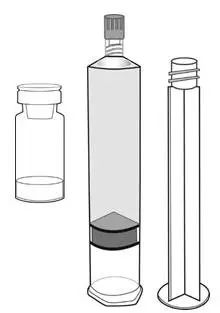 | 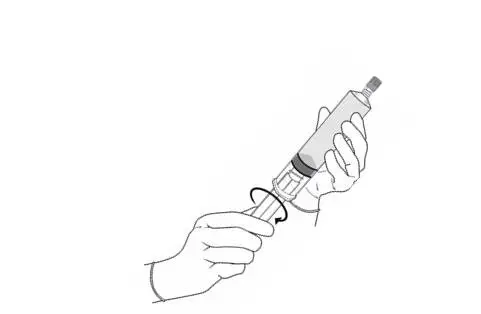 |
||
| Cysview Powder | Cysview Diluent | Plunger Rod | |
Figure 1.
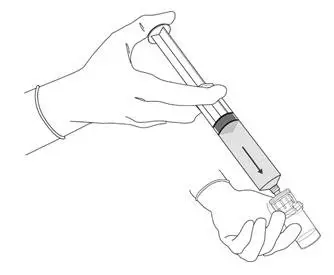
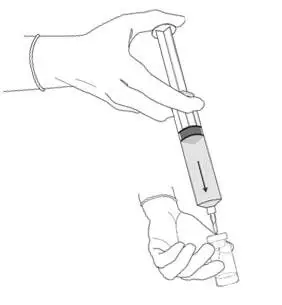
2.3 Bladder Instillation of Cysview
For bladder instillation of the solution of Cysview, use straight or intermittent, urethral catheters with a proximal funnel opening that will accommodate the Luer Lock adapter. Use only catheters made of vinyl (uncoated or coated with hydrogel), latex (amber or red), and silicone to instill the reconstituted Cysview. Do not use catheters coated or embedded with silver or antibiotics. In-dwelling bladder catheters (Foley catheters) may be used if the catheters are inserted shortly prior to Cysview administration and are removed following the Cysview instillation.
Use the following steps for bladder instillation of Cysview:
- Using standard sterile catheterization technique, first insert the urethral catheter into the bladder of the patient and use the catheter to completely empty the patient's bladder before instillation of Cysview.
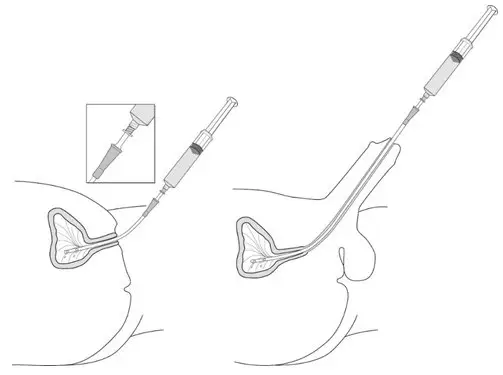
Figure 11.
- To attach the syringe containing the solution of Cysview to the catheter, do the following:
- Remove the syringe cap from the syringe that contains the reconstituted solution of Cysview.
- Attach the Luer Lock end of the (provided) catheter adapter to the syringe.
- Insert the tapered end of the catheter adapter into the funnel opening of the catheter. See Figure 11, with the connection enlarged in the inset.
- Slowly instill the solution of Cysview into the bladder through the catheter (Figure 11), ensuring that the complete volume of the syringe (50 mL) is administered.
- After the solution is instilled, remove the catheter and instruct the patient to retain the solution within the bladder for at least 1 hour; do not exceed 3 hours [ see Cystoscopic examination (2.5)]. Patients may stand, sit and move about during the time period between instillation and start of the cystoscopic procedure.
- Evacuate the solution of Cysview from the bladder as part of routine emptying of the bladder immediately prior to the initiation of the cystoscopic procedure (refer to the Karl Storz D-Light C Photodynamic Diagnostic (PDD) System Manual for further details). Also, the patient may void and completely empty the bladder prior to the procedure.
Avoid skin contact with Cysview. If skin does come in contact with Cysview, wash immediately with soap and water and dry off. After voiding the bladder of Cysview, routinely wash the patient's perineal skin region with soap and water and dry.
2.4 Use of the Karl Storz D-Light C Photodynamic Diagnostic (PDD) System
Cysview imaging requires the use of the Karl Storz D-Light C PDD system, which consists of either:
- a light source, a camera head, a camera control unit, a light cable, and a rigid cystoscope for use with the Rigid PDD Cystoscope System, or
- a light source, a camera control unit, and a flexible video cystoscope for use with the Flexible PDD Video Cystoscope System.
The light source enables both white light cystoscopy and blue light (wavelength 360 – 450 nm) fluorescence cystoscopy. Familiarity with this system is essential before beginning the procedure and before instilling Cysview into the bladder. For system set-up and general information for the safe use of the PDD system, refer to the Karl Storz instruction manual for the PDD system and the instruction manuals for each of the system components. The PDD System is not for use by healthcare providers with green-red color blindness.
3. Dosage Forms and Strengths
Cysview (hexaminolevulinate hydrochloride) is supplied as a kit. The kit may be supplied as two options; with or without a vial adapter, and contains:
4. Contraindications
Cysview is contraindicated in patients with:
- porphyria,
- gross hematuria,
- known hypersensitivity to hexaminolevulinate or any derivative of aminolevulinic acid.
5. Warnings and Precautions
5.1 Anaphylaxis
Anaphylaxis, including anaphylactoid shock, has been reported following administration of Cysview [ see Adverse Reactions (6.2)]. Prior to and during use of the Cysview, have trained personnel and therapies available for the treatment of anaphylaxis.
5.2 Failed Detection
Cysview may fail to detect some bladder tumors, including malignant lesions. Cysview is not a replacement for random biopsies or any other procedure usually performed in the cystoscopic evaluation for cancer. Do not perform cystoscopy with blue light alone as malignant lesions can be missed unless the bladder is initially examined under white light [ see Dosage and Administration (2.5) and Clinical Studies (14)].
5.3 False Positive Fluorescence
Fluorescent areas detected during blue light cystoscopy may not indicate a bladder mucosal lesion. In the controlled clinical studies, approximately 20% of the lesions detected only by blue light cystoscopy showed neither dysplasia nor carcinoma [ see Clinical Studies (14)]. False positive fluorescence may result from inflammation, cystoscopic trauma, scar tissue or bladder mucosal biopsy from a previous cystoscopic examination, and recent BCG immunotherapy or intravesical chemotherapy. In a study of patients treated with recent BCG immunotherapy or intravesical chemotherapy, the rate of false positives with blue light was 55% between 6 weeks to 90 days and 41% after 90 days; the false positive rate was 53% and 33% at the respective time intervals with white light.
The presence of urine and/or blood within the bladder may interfere with the detection of tissue fluorescence. To enhance the diagnostic utility of Cysview with the Karl Storz D-Light C PDD System:
- ensure the bladder is emptied of urine prior to the instillation of fluids at cystoscopy;
- biopsy/resect bladder mucosal lesions only following completion of both white light and blue light rigid cystoscopy;
6. Adverse Reactions/Side Effects
Anaphylaxis has been reported following exposure to Cysview [ see Warnings and Precautions (5.1)].
6.1 Clinical Study Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In seven clinical trials, safety data were obtained from 1,628 patients, aged 32 to 96 years with a median age of 70 years, all primarily Caucasian and approximately 75% male. All patients were evaluated after a single instillation of 50 mL solution of Cysview, and 103 patients received a repeat administration of Cysview. Of these patients, 170 (10.4%) patients reported at least one adverse reaction. The most common adverse reaction was bladder spasm (reported in 2.0% of the patients) followed by dysuria, hematuria, and bladder pain. No patients experienced anaphylaxis. In the randomized controlled clinical study, adverse reactions were similar in nature and rate between the study drug group and the control group. In a controlled study using Cysview in the surveillance setting, adverse reaction types were similar [ see Clinical Studies (14)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Cysview. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylactoid shock, hypersensitivity reactions, bladder pain, cystitis and abnormal urinalysis have been reported during post-marketing use of Cysview.
10. Overdosage
No adverse events were reported in a dose-finding study conducted among patients whose bladders were instilled with twice the recommended concentration (dose) of solution of Cysview.
11. Cysview Description
Cysview contains hexaminolevulinate hydrochloride, an optical imaging drug that in solution form is instilled intravesically for use with photodynamic blue light cystoscopy as an adjunct to white light cystoscopy.
The chemical formula for hexaminolevulinate hydrochloride is C 11H 21NO 3∙HCl. Its molecular weight is 251.76 and it has the following structural formula:
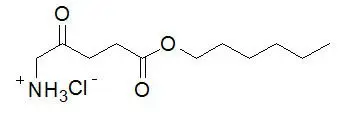
Cysview (hexaminolevulinate hydrochloride) for Intravesical Solution is intended for intravesical administration only after reconstitution with the supplied 50 mL DILUENT. Cysview (hexaminolevulinate hydrochloride) for Intravesical Solution and DILUENT for Cysview are supplied together as a kit.
Cysview (hexaminolevulinate hydrochloride) for Intravesical Solution is supplied as a sterile, non-pyrogenic, freeze-dried, white to off-white or pale yellow, powder containing 100 mg of hexaminolevulinate hydrochloride (equivalent of 85 mg of hexaminolevulinate) in a 10 mL clear glass vial. The DILUENT for Cysview is a sterile, non-pyrogenic solution (pH 6) containing 0.61 mg/ mL disodium hydrogen phosphate, 0.58 mg/mL of potassium dihydrogen phosphate, 7.02 mg/mL of sodium chloride, hydrochloric acid, sodium hydroxide, and water for injection. It is a clear, colorless solution, free from visible particles, and is provided in a 50 mL plastic prefilled syringe.
The reconstituted solution of Cysview contains 2 mg/ml of hexaminolevulinate hydrochloride and is colorless to pale yellow. It is free from visible particles and has a pH between 5.7 and 6.2.
12. Cysview - Clinical Pharmacology
12.1 Mechanism of Action
Cysview is an ester of the heme precursor, aminolevulinic acid. After bladder instillation, Cysview enters the bladder mucosa and is proposed to enter the intracellular space of mucosal cells where it is used as a precursor in the formation of the photoactive intermediate protoporphyrin IX (PpIX) and other photoactive porphyrins (PAPs). PpIX and PAPs are reported to accumulate preferentially in neoplastic cells as compared to normal urothelium, partly due to altered enzymatic activity in the neoplastic cells. After excitation with light at wavelengths between 360 and 450 nm, PpIX and other PAPs return to a lower energy level by fluorescing, which can be detected and used for cystoscopic detection of lesions. The fluorescence from tumor tissue appears bright red and demarcated, whereas the background normal tissue appears dark blue. Similar processes may occur in inflamed cells.
12.2 Pharmacodynamics
In vitro studies have shown increased porphyrin fluorescence in normal urothelium after exposure to Cysview. In the human bladder, a greater accumulation of porphyrins is proposed in neoplastic or inflamed cells, compared to normal urothelium. After bladder instillation of Cysview for approximately 1 hour and subsequent illumination with blue light at wavelengths 360 – 450nm, the porphyrins will fluoresce red [ see Dosage and Administration (2.5)] .
12.3 Pharmacokinetics
After bladder instillation of [ 14C]-labeled Cysview (100 mg) for approximately 1 hour in healthy volunteers, absolute bioavailability of Cysview was 7% (90% confidence interval [CI]: 5%-10%). The [ 14C]-labeled substance(s) showed biphasic elimination, with an initial elimination half-life of 39 minutes, followed by a terminal half-life of approximately 76 hours. Whole blood analysis showed no evidence of significant binding of Cysview to erythrocytes. An in vitro study showed that Cysview underwent rapid metabolism in human blood.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies in animals have been conducted to evaluate the carcinogenic potential of hexaminolevulinate hydrochloride.
Hexaminolevulinate hydrochloride was not mutagenic in in vitro reverse mutation tests in bacteria, or in chromosome aberration tests in human peripheral blood lymphocytes, and was negative in an in vivo micronucleus test in mice after intravenous injection of doses up to 45 mg/kg in the absence of light activation. Adequate studies have not been performed to evaluate the genetic toxicity of hexaminolevulinate hydrochloride in the presence of light activation.
Adequate reproductive and developmental toxicity studies in animals have not been performed to evaluate the effects of hexaminolevulinate hydrochloride on fertility.
13.2 Animal Toxicology and/or Pharmacology
Dose dependent neurological effects such as tremor, increased motor activity, and increased startle and touch escape responses were observed immediately after dosing at doses ≥ 30 mg/kg (24 times human systemic exposure based on the body surface area, using 10% as the upper level of 90% confidence interval of bioavailability) in a single-dose rat study. The animals recovered to normal status by 60 min after dosing. Adverse neurological effects were also noted in other single- or repeat-dose toxicity studies.
Hexaminolevulinate hydrochloride had moderate to strong potential to cause skin sensitization based on a local lymph node assay in mouse.
14. Clinical Studies
The safety and efficacy of Cysview when used with photodynamic cystoscopy were studied in two controlled clinical trials.
Study 1: A prospective, multicenter, controlled clinical trial in adult patients with known or suspected bladder cancer who were randomized to either white light (WL) cystoscopy (control group, n = 384) or WL followed by blue light (BL) cystoscopy (study drug group, n = 395). Only the study drug group patients received Cysview by bladder instillation prior to cystoscopy. After bladder evacuation of Cysview, bladder lesion mapping was performed initially using the Karl Storz PDD system in the WL mode followed by lesion mapping in the BL mode. Control group patients underwent only WL cystoscopy with lesion mapping. The average age of the randomized patients was 69 years (range 24 to 96); 78% were male and 94% were Caucasian. All patients had previously undergone cystoscopy.
The main diagnostic efficacy outcome was assessed within the study drug group. This assessment compared lesions detected during an initial cystoscopic examination to their centralized histologic findings (the standard of truth). Following the initial diagnostic cystoscopy, patients within both study groups who had histologically confirmed Ta and/or T1 lesions underwent follow-up WL cystoscopy at 3, 6 and 9 months; these histologic evaluations were based upon the site assessments at both the initial and follow-up cystoscopy.
Diagnostic efficacy assessed the number of patients within the study drug group who had at least one additional Ta or T1 bladder cancer detected only by BL; the proportion of these patients was compared to a proposed threshold proportion of 10%. Within the study drug group, 286 patients had at least one Ta and/or T1 lesion, including 47 patients who had at least one of the lesions detected only by BL (see Table 1).
|
|
| Number of patients with any Ta and/or T1 lesion detected with either WL or BL | 286 |
| Number (%) of patients with any Ta and/or T1 lesion detected only with BL | 47 (16%) |
| p-value * | 0.001 |
Some malignant lesions were detected only by WL or BL (see Table 2).
| Number of lesions | Detected by Both WL & BL | Detected by WL Only | Detected by BL Only |
|---|---|---|---|
| CIS, n = 66 | 33 | 6 | 27 |
| Ta, n = 580 | 472 | 52 | 56 |
| T1, n = 95 | 76 | 10 | 9 |
| T2 – T4, n = 47 | 38 | 8 | 1 |
Among the lesions detected only by BL, 23% were negative for any carcinoma-related pathology, including dysplasia. Among the lesions detected only by WL, 17% were negative for any carcinoma-related pathology, including dysplasia.
Study 2: A prospective, open-label, within-patient controlled clinical trial using BL cystoscopy in the detection of bladder cancer during surveillance cystoscopy. Patients with bladder cancer in follow-up for tumor recurrence (n=304) received Cysview by bladder instillation. The average age of the patients was 69 years (range 35 to 92); 80% were male and 89% were Caucasian. After bladder evacuation of Cysview, a standard WL cystoscopy was performed, followed by BL cystoscopy using the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) System with the Flexible PDD Videoscope System. Suspected malignant lesions were counted and evaluated. Patients with suspected recurrence (n=103), underwent a Cysview instillation followed by WL and BL rigid cystoscopy in the operating room (OR), including lesion mapping, using the KARL STORZ D-Light C PDD System with the Rigid PDD Cystoscope System. The suspicious lesions were biopsied and surgically removed by TURB. Cysview efficacy assessed the proportion of patients with malignancy detected only with blue light cystoscopy and not WL cystoscopy during the surveillance cystoscopic examination. The assessment was performed at patient level, and compared malignancy detected during the surveillance cystoscopic examination to the centralized histologic findings (the standard of truth) obtained in the OR examination.
Table 3 shows patient-level detection of malignancy suspected in cystoscopic surveillance stage that was verified in the OR stage (n=103). Among the 103 patients, 63 patients had malignancy confirmed: 49 patients had malignancy detected by both WL and BL; 1 patient had malignancy detected by WL only; and 13 patients had malignancy detected by BL only [12.6% with 95% CI (7%, 21%), p<0.0001*]. Among these 103 patients, 40 patients had false positive detections: 17 patients had false positive detection by both WL and BL; 3 patients had false positive detection by WL only; and 20 patients had false positive detection by BL only.
| Detected by Both WL and BL | Detected by WL only | Detected by BL only | Total | |
|---|---|---|---|---|
| * Exact test comparison of the proportion to a threshold value of 0.5% | ||||
| True Positive | 49 | 1 | 13 | 63 |
| False Positive | 17 | 3 | 20 | 40 |
| Total | 66 | 4 | 33 | 103 |
Among 26 patients with confirmed CIS malignancy, 9 patients had CIS malignancy detected by BL only and 17 patients had CIS malignancy detected by both WL and BL.
In the same study, there were 315 lesions detected during the cystoscopy in the OR. Table 4 shows the detection of lesions by type of malignancy.
| Malignancy Type | Detected by Both WL & BL | Detected by WL Only | Detected by BL Only | ||
|---|---|---|---|---|---|
|
|||||
| CIS, n = 43 | 24 | 3 | 16 | ||
| Ta, n = 94 | 61 | 9 | 24 | ||
| T1, n = 10 | 7 | 0 | 3 | ||
| T2 – T4, n = 5 | 5 | 0 | 0 | ||
| PUNLMP * n=3 | 2 | 0 | 1 | ||
| False positive n=160 | 65 | 22 | 73 | ||
| Total number of lesions | 164 | 34 | 117 | ||
16. How is Cysview supplied
Cysview is supplied as a kit labeled Cysview (hexaminolevulinate HCl) Kit for Intravesical Solution, 100 mg. The kit may be supplied as two options; with or without a vial adapter, and contains:
17. Patient Counseling Information
Ask patients if they have:
- a diagnosis or a family history of porphyria,
- allergy to aminolevulinic acid or prior exposure to Cysview,
- gross hematuria,
- had BCG immunotherapy or chemotherapy within the bladder.
Inform patients that Cysview should be retained in the bladder for 1 hour from instillation of Cysview to the start of the cystoscopic procedure. If the patient cannot hold Cysview for 1 hour but needs to void and expel Cysview from the bladder, he or she may void and should then inform a healthcare professional [ see Dosage and Administration (2)].
| CYSVIEW
hexaminolevulinate hydrochloride kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Photocure Inc. (006629060) |