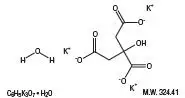
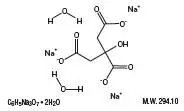
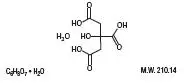
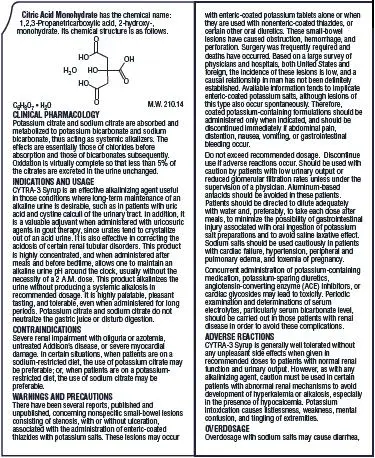
Drug Detail:Cytra-k (Citric acid and potassium citrate [ sit-rik-as-id-and-poe-tass-ee-um-si-trate ])
Drug Class: Minerals and electrolytes
| CYTRA
3
potassium citrate monohydrate, sodium citrate dihydrate, citric acid monohydrate syrup |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Cypress Pharmaceuticals, Inc (790248942) |