Drug Detail:Darzalex faspro (Daratumumab and hyaluronidase-fihj)
Drug Class: CD38 monoclonal antibodies
Highlights of Prescribing Information
DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) injection, for subcutaneous use
Initial U.S. Approval: 2020
Recent Major Changes
| Dosage and Administration ( 2.7) | 4/2022 |
| Warnings and Precautions ( 5.1) | 1/2022 |
Indications and Usage for Darzalex Faspro
DARZALEX FASPRO is a combination of daratumumab, a CD38-directed cytolytic antibody, and hyaluronidase, an endoglycosidase, indicated for the treatment of adult patients with:
- multiple myeloma in combination with bortezomib, melphalan and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
- multiple myeloma in combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy
- multiple myeloma in combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant
- multiple myeloma in combination with bortezomib and dexamethasone in patients who have received at least one prior therapy
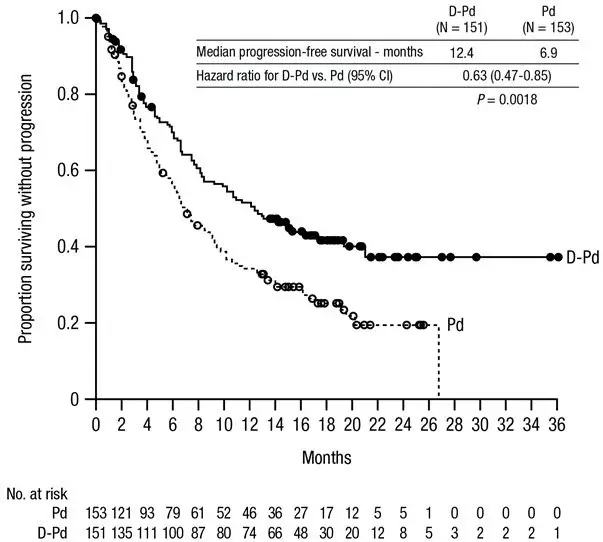
- multiple myeloma in combination with pomalidomide and dexamethasone in patients who have received at least one prior line of therapy including lenalidomide and a proteasome inhibitor
- multiple myeloma in combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy
- multiple myeloma as monotherapy, in patients who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent
- light chain (AL) amyloidosis in combination with bortezomib, cyclophosphamide and dexamethasone in newly diagnosed patients. This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s) ( 1.2)
Limitations of Use:
- DARZALEX FASPRO is not indicated and is not recommended for the treatment of patients with light chain (AL) amyloidosis who have NYHA Class IIIB or Class IV cardiac disease or Mayo Stage IIIB outside of controlled clinical trials ( 1.2)
Darzalex Faspro Dosage and Administration
For subcutaneous use only.
- Pre-medicate with a corticosteroid, acetaminophen and a histamine-1 receptor antagonist. ( 2.5)
- The recommended dosage of DARZALEX FASPRO is (1,800 mg daratumumab and 30,000 units hyaluronidase) administered subcutaneously into the abdomen over approximately 3 to 5 minutes according to recommended schedule. ( 2.2, 2.3)
- Administer post-medications as recommended. ( 2.5)
Dosage Forms and Strengths
- Injection: 1,800 mg daratumumab and 30,000 units hyaluronidase per 15 mL (120 mg and 2,000 units/mL) solution in a single-dose vial ( 3)
Contraindications
Patients with a history of severe hypersensitivity to daratumumab, hyaluronidase or any of the components of the formulation. ( 4)
Warnings and Precautions
- Hypersensitivity and Other Administration Reactions: Permanently discontinue DARZALEX FASPRO for life-threatening reactions. ( 5.1)
- Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis: Monitor patients with cardiac involvement more frequently for cardiac adverse reactions and administer supportive care as appropriate. ( 5.2)
- Neutropenia: Monitor complete blood cell counts periodically during treatment. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX FASPRO to allow recovery of neutrophils. ( 5.3)
- Thrombocytopenia: Monitor complete blood cell counts periodically during treatment. Consider withholding DARZALEX FASPRO to allow recovery of platelets. ( 5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise pregnant women of the potential risk to a fetus and advise females of reproductive potential to use effective contraception. ( 5.5, 8.1, 8.3)
- Interference with cross-matching and red blood cell antibody screening: Type and screen patients prior to starting treatment. Inform blood banks that a patient has received DARZALEX FASPRO. ( 5.6, 7.1)
Adverse Reactions/Side Effects
- The most common adverse reaction (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO monotherapy is upper respiratory tract infection. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-VMP are upper respiratory tract infection, constipation, nausea, fatigue, pyrexia, peripheral sensory neuropathy, diarrhea, cough, insomnia, vomiting, and back pain. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-Rd are fatigue, diarrhea, upper respiratory tract infection, muscle spasms, constipation, pyrexia, pneumonia, and dyspnea. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-Pd are fatigue, pneumonia, upper respiratory tract infection, and diarrhea. ( 6.1)
- The most common adverse reactions (≥20%) in patients with multiple myeloma who received DARZALEX FASPRO-Kd are upper respiratory tract infection, fatigue, insomnia, hypertension, diarrhea, cough, dyspnea, headache, pyrexia, nausea, and edema peripheral. ( 6.1)
- The most common adverse reactions (≥20%) in patients with light chain (AL) amyloidosis are upper respiratory tract infection, diarrhea, peripheral edema, constipation, fatigue, peripheral sensory neuropathy, nausea, insomnia, dyspnea, and cough. ( 6.1)
- The most common (≥40%) hematology laboratory abnormalities with DARZALEX FASPRO are decreased leukocytes, decreased lymphocytes, decreased neutrophils, decreased platelets, and decreased hemoglobin. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2022
Full Prescribing Information
1. Indications and Usage for Darzalex Faspro
1.1 Multiple Myeloma
DARZALEX FASPRO is indicated for the treatment of adult patients with multiple myeloma:
- in combination with bortezomib, melphalan and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant.
- in combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy.
- in combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant.
- in combination with bortezomib and dexamethasone in patients who have received at least one prior therapy.
- in combination with pomalidomide and dexamethasone in patients who have received at least one prior line of therapy including lenalidomide and a proteasome inhibitor.
- in combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy.
- as monotherapy, in patients who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent.
1.2 Light Chain Amyloidosis
DARZALEX FASPRO in combination with bortezomib, cyclophosphamide and dexamethasone is indicated for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis.
This indication is approved under accelerated approval based on response rate [see Clinical Studies (14.3)] . Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
2. Darzalex Faspro Dosage and Administration
2.1 Important Dosing Information
- DARZALEX FASPRO is for subcutaneous use only.
- Administer medications before and after administration of DARZALEX FASPRO to minimize administration-related reactions [see Dosage and Administration (2.5)] .
- Type and screen patients prior to starting DARZALEX FASPRO.
2.2 Recommended Dosage for Multiple Myeloma
The recommended dose of DARZALEX FASPRO is 1,800 mg/30,000 units (1,800 mg daratumumab and 30,000 units hyaluronidase) administered subcutaneously over approximately 3–5 minutes. Tables 1, 2, 3, and 4 provide the recommended dosing schedule when DARZALEX FASPRO is administered as monotherapy or as part of a combination therapy.
2.4 Administration
If a dose of DARZALEX FASPRO is missed, administer the dose as soon as possible and adjust the dosing schedule to maintain the dosing interval.
2.6 Dosage Modifications for Adverse Reactions
No dose reductions of DARZALEX FASPRO are recommended. Consider withholding DARZALEX FASPRO to allow recovery of blood cell counts in the event of myelosuppression [see Warnings and Precautions (5.3, 5.4)] .
2.7 Preparation and Administration
DARZALEX FASPRO should be administered by a healthcare provider.
To prevent medication errors, check the vial labels to ensure that the drug being prepared and administered is DARZALEX FASPRO for subcutaneous use. Do not administer DARZALEX FASPRO intravenously.
DARZALEX FASPRO is ready to use.
3. Dosage Forms and Strengths
Injection: 1,800 mg daratumumab and 30,000 units hyaluronidase per 15 mL (120 mg and 2,000 units/mL) colorless to yellow and clear to opalescent solution in a single-dose vial.
4. Contraindications
DARZALEX FASPRO is contraindicated in patients with a history of severe hypersensitivity to daratumumab, hyaluronidase or any of the components of the formulation [see Warnings and Precautions (5.1) and Adverse Reactions (6.3)].
5. Warnings and Precautions
5.1 Hypersensitivity and Other Administration Reactions
Both systemic administration-related reactions, including severe or life-threatening reactions, and local injection-site reactions can occur with DARZALEX FASPRO. Fatal reactions have been reported with daratumumab-containing products, including DARZALEX FASPRO [see Adverse Reactions (6.3)] .
5.2 Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis
Serious or fatal cardiac adverse reactions occurred in patients with light chain (AL) amyloidosis who received DARZALEX FASPRO in combination with bortezomib, cyclophosphamide and dexamethasone [see Adverse Reactions (6.1)] . Serious cardiac disorders occurred in 16% and fatal cardiac disorders occurred in 10% of patients. Patients with NYHA Class IIIA or Mayo Stage IIIA disease may be at greater risk. Patients with NYHA Class IIIB or IV disease were not studied.
Monitor patients with cardiac involvement of light chain (AL) amyloidosis more frequently for cardiac adverse reactions and administer supportive care as appropriate.
5.3 Neutropenia
Daratumumab may increase neutropenia induced by background therapy [see Adverse Reactions (6.1)] .
Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX FASPRO until recovery of neutrophils. In lower body weight patients receiving DARZALEX FASPRO, higher rates of Grade 3–4 neutropenia were observed.
5.4 Thrombocytopenia
Daratumumab may increase thrombocytopenia induced by background therapy [see Adverse Reactions (6.1)] .
Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Consider withholding DARZALEX FASPRO until recovery of platelets.
5.5 Embryo-Fetal Toxicity
Based on the mechanism of action, DARZALEX FASPRO can cause fetal harm when administered to a pregnant woman. DARZALEX FASPRO may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX FASPRO and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)] .
The combination of DARZALEX FASPRO with lenalidomide, thalidomide or pomalidomide is contraindicated in pregnant women, because lenalidomide, thalidomide or pomalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, thalidomide or pomalidomide prescribing information on use during pregnancy.
5.6 Interference with Serological Testing
Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive Indirect Antiglobulin Test (Indirect Coombs test). Daratumumab-mediated positive indirect antiglobulin test may persist for up to 6 months after the last daratumumab administration. Daratumumab bound to RBCs masks detection of antibodies to minor antigens in the patient's serum [see References (15)] . The determination of a patient's ABO and Rh blood type are not impacted [see Drug Interactions (7.1)] .
Notify blood transfusion centers of this interference with serological testing and inform blood banks that a patient has received DARZALEX FASPRO. Type and screen patients prior to starting DARZALEX FASPRO [see Dosage and Administration (2.1)] .
5.7 Interference with Determination of Complete Response
Daratumumab is a human IgG kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein [see Drug Interactions (7.1)] . This interference can impact the determination of complete response and of disease progression in some DARZALEX FASPRO-treated patients with IgG kappa myeloma protein.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity and Other Administration Reactions [see Warnings and Precautions (5.1)] .
- Cardiac Toxicity in Patients with Light Chain (AL) Amyloidosis [see Warnings and Precautions (5.2)] .
- Neutropenia [see Warnings and Precautions (5.3)] .
- Thrombocytopenia [see Warnings and Precautions (5.4)] .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Immunogenicity
As with all therapeutic proteins, there is the potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other daratumumab products or other hyaluronidase products may be misleading.
In patients with multiple myeloma and light chain (AL) amyloidosis who received DARZALEX FASPRO as monotherapy or as part of a combination therapy, less than 1% of 819 patients developed treatment-emergent anti-daratumumab antibodies.
In patients with multiple myeloma and light chain (AL) amyloidosis who received DARZALEX FASPRO as monotherapy or as part of a combination therapy, 7% of 812 patients developed treatment-emergent anti-rHuPH20 antibodies. The anti-rHuPH20 antibodies did not appear to affect daratumumab exposure. None of the patients who tested positive for anti-rHuPH20 antibodies tested positive for neutralizing antibodies.
6.3 Postmarketing Experience
The following adverse reactions have been identified with post-approval use of daratumumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System: Anaphylactic reaction, Systemic administration reactions (including death)
Gastrointestinal: Pancreatitis
Infections: Cytomegalovirus, Listeriosis
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
DARZALEX FASPRO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] .
8.4 Pediatric Use
Safety and effectiveness of DARZALEX FASPRO in pediatric patients have not been established.
8.5 Geriatric Use
Of the 291 patients who received DARZALEX FASPRO as monotherapy for relapsed and refractory multiple myeloma, 37% were 65 to <75 years of age, and 19% were 75 years of age or older. No overall differences in effectiveness of DARZALEX FASPRO have been observed between patients ≥65 years of age and younger patients . Adverse reactions that occurred at a higher frequency (≥5% difference) in patients ≥65 years of age included upper respiratory tract infection, urinary tract infection, dizziness, cough, dyspnea, diarrhea, nausea, fatigue, and peripheral edema. Serious adverse reactions that occurred at a higher frequency (≥2% difference) in patients ≥65 years of age included pneumonia.
Of the 214 patients who received DARZALEX FASPRO as combination therapy with pomalidomide and dexamethasone or DARZALEX FASPRO as combination therapy with lenalidomide and low-dose dexamethasone for relapsed and refractory multiple myeloma, 43% were 65 to <75 years of age, and 18% were 75 years of age or older. No overall differences in effectiveness were observed between patients ≥65 years (n=131) and <65 years (n=85). Adverse reactions occurring at a higher frequency (≥5% difference) in patients ≥65 years of age included fatigue, pyrexia, peripheral edema, urinary tract infection, diarrhea, constipation, vomiting, dyspnea, cough, and hyperglycemia. Serious adverse reactions occurring at a higher frequency (≥2% difference) in patients ≥65 years of age included neutropenia, thrombocytopenia, diarrhea, anemia, COVID-19, ischemic colitis, deep vein thrombosis, general physical health deterioration, pulmonary embolism, and urinary tract infection.
Of the 193 patients who received DARZALEX FASPRO as part of a combination therapy for light chain (AL) amyloidosis, 35% were 65 to <75 years of age, and 10% were 75 years of age or older. Clinical studies of DARZALEX FASPRO as part of a combination therapy for patients with light chain (AL) amyloidosis did not include sufficient numbers of patients aged 65 and older to determine whether effectiveness differs from that of younger patients. Adverse reactions that occurred at a higher frequency in patients ≥65 years of age were peripheral edema, asthenia, pneumonia and hypotension.
No clinically meaningful differences in the pharmacokinetics of daratumumab were observed in geriatric patients compared to younger adult patients [see Clinical Pharmacology (12.3)] .
11. Darzalex Faspro Description
Daratumumab is an immunoglobulin G1 kappa (IgG1κ) human monoclonal antibody that binds to the CD38 antigen. Daratumumab is produced in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology. The molecular weight of daratumumab is approximately 148 kDa.
Hyaluronidase (recombinant human) is an endoglycosidase used to increase the dispersion and absorption of co-administered drugs when administered subcutaneously. It is a glycosylated single-chain protein produced by Chinese Hamster Ovary cells containing a DNA plasmid encoding for a soluble fragment of human hyaluronidase (PH20). Hyaluronidase (recombinant human) has a molecular weight of approximately 61 kDa.
DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) injection is a sterile, preservative-free, colorless to yellow, and clear to opalescent solution supplied in a single-dose vial for subcutaneous administration.
Each DARZALEX FASPRO 15 mL single-dose vial contains 1,800 mg of daratumumab and 30,000 units of hyaluronidase, L-histidine (4.9 mg), L-histidine hydrochloride monohydrate (18.4 mg), L-methionine (13.5 mg), polysorbate 20 (6 mg), sorbitol (735.1 mg), and Water for Injection, USP.
12. Darzalex Faspro - Clinical Pharmacology
12.1 Mechanism of Action
CD38 is a transmembrane glycoprotein (48 kDa) expressed on the surface of hematopoietic cells, including clonal plasma cells in multiple myeloma and light chain (AL) amyloidosis, as well as other cell types. Surface CD38 has multiple functions, including receptor mediated adhesion, signaling, and modulation of cyclase and hydrolase activity. Daratumumab is an IgG1κ human monoclonal antibody (mAb) that binds to CD38 and inhibits the growth of CD38 expressing tumor cells by inducing apoptosis directly through Fc mediated cross linking as well as by immune-mediated tumor cell lysis through complement dependent cytotoxicity (CDC), antibody dependent cell mediated cytotoxicity (ADCC) and antibody dependent cellular phagocytosis (ADCP). A subset of myeloid derived suppressor cells (CD38+MDSCs), regulatory T cells (CD38+T regs) and B cells (CD38+B regs) are decreased by daratumumab.
Hyaluronan is a polysaccharide found in the extracellular matrix of the subcutaneous tissue. It is depolymerized by the naturally occurring enzyme hyaluronidase. Unlike the stable structural components of the interstitial matrix, hyaluronan has a half-life of approximately 0.5 days. Hyaluronidase increases permeability of the subcutaneous tissue by depolymerizing hyaluronan. In the doses administered, hyaluronidase in DARZALEX FASPRO acts locally. The effects of hyaluronidase are reversible and permeability of the subcutaneous tissue is restored within 24 to 48 hours.
12.2 Pharmacodynamics
NK cells express CD38 and are susceptible to daratumumab mediated cell lysis. Decreases in absolute counts and percentages of total NK cells (CD16+CD56+) and activated (CD16+CD56 dim) NK cells in peripheral whole blood and bone marrow were observed with DARZALEX FASPRO treatment.
12.3 Pharmacokinetics
Following the recommended dose of DARZALEX FASPRO 1,800 mg/30,000 units subcutaneously once weekly for 8 weeks, daratumumab peak concentration (C max) increased 4.8-fold and area under the curve (AUC 0–7 days) increased 5.4-fold from the 1 st dose to the 8 th dose as monotherapy. Maximum trough concentrations for DARZALEX FASPRO are typically observed at the end of the weekly dosing regimens for both monotherapy and combination therapies. The mean ± standard deviation (SD) maximum trough serum concentration (C trough) after the 8 th dose was 593 ± 306 µg/mL when DARZALEX FASPRO was administered as monotherapy and 537 ± 277 µg/mL, 526 ± 226 µg/mL, and 756 ± 276 µg/mL when DARZALEX FASPRO was administered as combination with Pd, Rd, and Kd, respectively.
Table 18 lists the observed mean (±SD) maximum trough concentrations (C trough) after the 8 th dose, simulated median (5 th–95 th percentiles) maximum C trough after the 8 th dose, simulated median (5 th–95 th percentiles) C max after the 8 th dose, and simulated median (5 th–95 th percentiles) area under the curve (AUC 0–7day) after the 8 th dose following DARZALEX FASPRO 1,800 mg/30,000 units administered subcutaneously or daratumumab 16 mg/kg administered intravenously in patients with multiple myeloma or light chain (AL) amyloidosis.
| Parameter | Intravenous Daratumumab 16 mg/kg in Patients with Multiple Myeloma * | DARZALEX FASPRO 1,800 mg/30,000 units in Patients with Multiple Myeloma * | DARZALEX FASPRO 1,800 mg/30,000 units in Patients with Light Chain (AL) Amyloidosis † |
|---|---|---|---|
|
|||
| Observed mean±SD max C trough after 8 th dose (µg/mL) | 522±226 ‡,§ | 593±306 ‡,§ | 597±232 ¶ |
| Simulated median (5 th–95 th percentiles) max C trough after 8 th dose (µg/mL) | 472 (144–809) # | 563 (177–1063) # | 662 (315–1037) Þ |
| Simulated median (5 th–95 th percentiles) C max after 8 th dose (µg/mL) | 688 (369–1061) # | 592 (234–1114) # | 729 (390–1105) Þ |
| Simulated median (5 th–95 th percentiles) AUC 0–7days after 8 th dose (µg/mL∙day) | 4019 (1740–6370) # | 4017 (1515–7564) # | 4855 (2562–7522) Þ |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with daratumumab. No animal studies have been performed to evaluate the potential effects of daratumumab on reproduction or development, or to determine potential effects on fertility in males or females.
No carcinogenicity, genotoxicity, or fertility studies were conducted for recombinant human hyaluronidase. There were no effects on reproductive tissues and function and no systemic exposure of hyaluronidase in monkeys given 22,000 U/kg/week subcutaneously (12 times higher than the human dose) for 39 weeks. As hyaluronidase is a recombinant form of the endogenous human hyaluronidase, no carcinogenicity, mutagenesis, or effects on fertility are expected.
14. Clinical Studies
15. References
- Chapuy, CI, RT Nicholson, MD Aguad, et al., 2015, Resolving the daratumumab interference with blood compatibility testing, Transfusion, 55:1545–1554 (accessible at http://onlinelibrary.wiley.com/doi/10.1111/trf.13069/epdf).
16. How is Darzalex Faspro supplied
DARZALEX FASPRO ® (daratumumab and hyaluronidase-fihj) injection is a sterile, preservative-free, colorless to yellow, and clear to opalescent solution for subcutaneous use supplied as individually packaged single-dose vials providing 1,800 mg of daratumumab and 30,000 units of hyaluronidase per 15 mL (NDC 57894-503-01).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
PRINCIPAL DISPLAY PANEL - 15 mL Vial Box
NDC 57894-503-01
DARZALEX Faspro
®
(daratumumab and
hyaluronidase-fihj)
Injection
1,800 mg and
30,000 Units/15 mL
(120 mg and 2,000 Units/mL)
For Subcutaneous Use Only
Administer subcutaneous
injection over 3 to 5 minutes.
Rx only
One 15 mL Vial
Single-dose vial.
Discard unused portion.
janssen
| DARZALEX FASPRO
daratumumab and hyaluronidase-fihj (human recombinant) injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Janssen Biotech, Inc. (099091753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Biotech, Inc. | 038978363 | api manufacture(57894-503) , analysis(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc | 069777290 | analysis(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Charles River Laboratories | 078495006 | analysis(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bioreliance Corporation | 147227730 | analysis(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Indiana, LLC | 172209277 | api manufacture(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biogen (Denmark) Manufacturing ApS | 307258082 | api manufacture(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Biologics B.V. | 409612918 | analysis(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cilag AG | 483237103 | manufacture(57894-503) , analysis(57894-503) , pack(57894-503) , label(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Vaccines Corp. | 688272061 | manufacture(57894-503) , pack(57894-503) , analysis(57894-503) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Sciences Ireland UC | 986030167 | analysis(57894-503) | |