Drug Detail:Dengue vaccine live (monograph) (Dengvaxia)
Drug Class:
Highlights of Prescribing Information
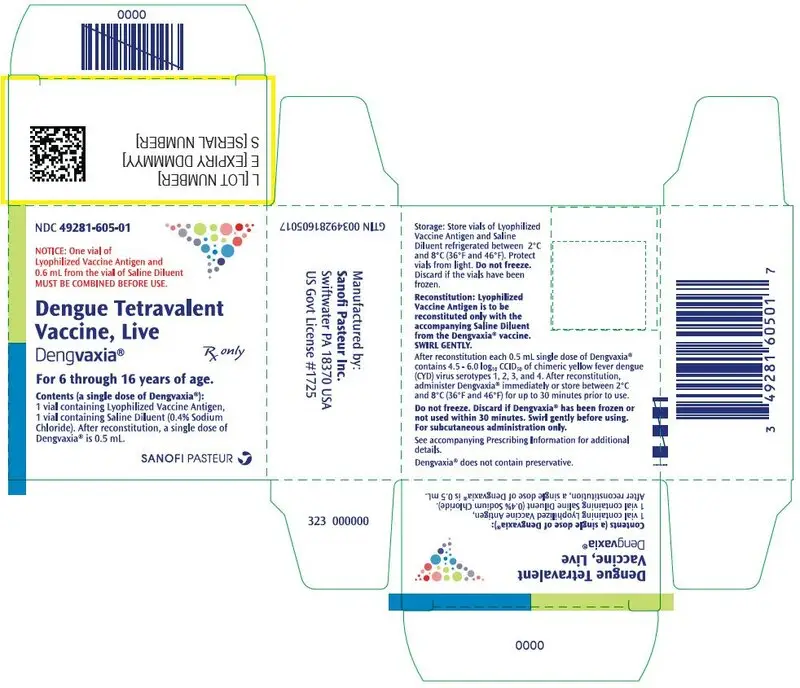
DENGVAXIA (Dengue Tetravalent Vaccine, Live)
Suspension for Subcutaneous Injection
Initial U.S. Approval: 2019
Recent Major Changes
| Indications and Usage (1) | 07/2023 |
| Warnings and Precautions (5.1) | 07/2023 |
Indications and Usage for Dengvaxia
DENGVAXIA is a vaccine indicated for the prevention of dengue disease caused by dengue virus serotypes 1, 2, 3 and 4. DENGVAXIA is approved for use in individuals 6 through 16 years of age with laboratory-confirmed previous dengue infection and living in endemic areas.
Limitations of use:
- DENGVAXIA is not approved for use in individuals younger than 6 years of age. These individuals, regardless of previous infection by dengue virus, are at increased risk of severe and hospitalized dengue disease following vaccination with DENGVAXIA and subsequent infection with any dengue virus serotype. (5.1)
- DENGVAXIA is not approved for use in individuals not previously infected by any dengue virus serotype or for whom this information is unknown. Those not previously infected are at increased risk for severe dengue disease when vaccinated and subsequently infected with dengue virus. (5.2) Previous dengue infection can be assessed through a medical record of a previous laboratory-confirmed dengue infection or through serological testing prior to vaccination. (1)
- The safety and effectiveness of DENGVAXIA have not been established in individuals living in dengue nonendemic areas who travel to dengue endemic areas. (1)
Dengvaxia Dosage and Administration
Three doses (0.5 mL each) 6 months apart (at month 0, 6, and 12). (2.1)
Dosage Forms and Strengths
Suspension for injection (0.5 mL) supplied as a lyophilized powder to be reconstituted with the supplied diluent. (3)
Contraindications
- A history of severe allergic reaction to a previous dose of DENGVAXIA or to any component of DENGVAXIA. (4.1)
- Immunocompromised individuals. (4.2)
Warnings and Precautions
- In persons younger than 6 years of age, regardless of previous infection by dengue virus, an increased risk of severe and hospitalized dengue disease can occur following vaccination with DENGVAXIA and subsequent infection with any dengue virus serotype. (5.1)
- In persons not previously infected by dengue virus, an increased risk of severe dengue disease can occur following vaccination with DENGVAXIA and subsequent infection with any dengue virus serotype. (5.2)
- There is no FDA-cleared test available to determine a previous dengue infection. (5.2)
Adverse Reactions/Side Effects
The most frequently reported adverse reactions regardless of the dengue serostatus prior to vaccination in ages 9 through 16 years were headache (40%), injection site pain (32%), malaise (25%), asthenia (25%), and myalgia (29%).
The most frequently reported adverse reactions regardless of the dengue serostatus prior to vaccination in ages 6 through8 years were headache (27%), injection site pain (28%), malaise (19%), asthenia (13%), and myalgia (15%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
Drug Interactions
False negative tuberculin purified protein derivative (PPD) test results may occur within 1 month following vaccination with DENGVAXIA. (7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
Full Prescribing Information
1. Indications and Usage for Dengvaxia
DENGVAXIA® (Dengue Tetravalent Vaccine, Live) is a vaccine indicated for the prevention of dengue disease caused by dengue virus serotypes 1, 2, 3, and 4. DENGVAXIA is approved for use in individuals 6 through 16 years of age with laboratory-confirmed previous dengue infection and living in endemic areas.
2. Dengvaxia Dosage and Administration
For subcutaneous use only.
2.2 Preparation
The package contains a vial of lyophilized vaccine antigen and a vial of saline diluent (0.4% NaCl).
After removing the "flip-off" caps, cleanse the lyophilized vaccine antigen and diluent vial stoppers with a suitable germicide. Do not remove the vial stoppers or metal seals holding them in place.
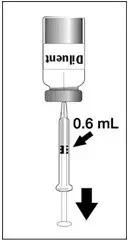
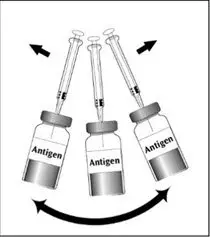
To reconstitute DENGVAXIA, use a sterile needle and syringe to withdraw 0.6 mL from the diluent vial and inject it into the vial of the lyophilized vaccine antigen. Swirl the vial gently.
Changing needles between withdrawing the vaccine from the vial and injecting it into a recipient is not necessary unless the needle has been damaged or contaminated.
 |  |  |  |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
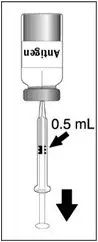
| Insert the syringe needle through the stopper of the vial of diluent and withdraw 0.6 mL liquid content. | Insert the syringe needle through the stopper of the vial of lyophilized vaccine antigen and inject the liquid into the vial. | Swirl vial gently. The syringe needle is not removed while swirling the vial. | After reconstitution, withdraw 0.5 mL. DENGVAXIA should be used immediately after reconstitution. |
After reconstitution, the suspension is colorless and may develop trace amounts of white to translucent endogenous proteinaceous particles. [See Description (11).]
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the vial if the solution is cloudy or contains particles other than trace amounts of white to translucent particles.
Discard reconstituted vaccine if not used within 30 minutes. [See How Supplied/Storage and Handling (16.2).]
DENGVAXIA should not be mixed in the same syringe with other parenteral products.
3. Dosage Forms and Strengths
DENGVAXIA is a suspension for injection (supplied as a lyophilized powder to be reconstituted with the supplied diluent, 0.4% NaCl). A single dose, after reconstitution, is 0.5 mL.
4. Contraindications
5. Warnings and Precautions
5.1 Increased Risk of Severe Dengue Disease Following DENGVAXIA in Persons Younger than 6 Years of Age Regardless of Previous Infection with Dengue Virus
DENGVAXIA is not approved for use in individuals younger than 6 years of age. In persons younger than 6 years of age regardless of previous infection by dengue virus, an increased risk of severe and hospitalized dengue disease can occur following vaccination with DENGVAXIA and subsequent infection with any dengue virus serotype. [see Use in Specific Populations (8.4)]
5.2 Increased Risk of Severe Dengue Disease Following DENGVAXIA in Persons of Any Age Not Previously Infected with Dengue Virus
In unvaccinated individuals, first dengue infections rarely cause severe dengue, while second dengue infections with a different serotype are associated with an increased risk of severe dengue. DENGVAXIA administration to individuals not previously infected by dengue virus is associated with an increased risk of severe dengue disease when the vaccinated individual is subsequently infected with any dengue virus serotype. Therefore, healthcare professionals must evaluate individuals for prior dengue infection to avoid vaccinating individuals who have not been previously infected by dengue virus.
Previous infection by dengue virus can be evaluated through a medical record of previous laboratory-confirmed dengue infection or through serotesting prior to vaccination.
There is no FDA cleared test available to determine a previous dengue infection. Available non-FDA cleared tests may yield false positive results (e.g., due to cross-reactivity with other flaviviruses).
5.3 Management of Acute Allergic Reactions
DENGVAXIA may cause hypersensitivity reactions, including anaphylaxis. Appropriate medical treatment and supervision must be available following administration of DENGVAXIA.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of DENGVAXIA in subjects 6 through 16 years of age was evaluated in 12 randomized, placebo-controlled, multicenter clinical trials with at least 28 days of follow-up conducted primarily in dengue endemic regions including Asia-Pacific and Latin American countries. In these studies, a total of 22,924 subjects 6 through 16 years of age received at least one dose of DENGVAXIA and 10,668 received placebo (0.9% sodium chloride). Overall, 51.6% of trial participants who received DENGVAXIA or placebo were female. Racial groups were reported as 27% Asian, 11.9% American Indian or Alaska native, 5.8% Caucasian, 2.3% black, and 53% as other. In the largest study (Study 1, NCT01374516; N = 20,869) conducted in four Latin American countries and Puerto Rico, most subjects (99.9%) reported Hispanic ethnicity. All studies enrolled subjects irrespective of evidence of previous dengue infection.
6.2 Postmarketing Experience
In addition to events reported in clinical trials for DENGVAXIA, the following adverse events have been spontaneously reported during postapproval use. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
The following adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to DENGVAXIA.
7. Drug Interactions
7.1 Immunosuppressive Treatments
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to DENGVAXIA.
7.2 Drug/Laboratory Test Interactions
DENGVAXIA may cause temporary depression of tuberculin purified protein derivative (PPD) test sensitivity, leading to false negative results. Tuberculin testing should be performed before DENGVAXIA is administered or at least 1 month following vaccination with DENGVAXIA.
8. Use In Specific Populations
8.4 Pediatric Use
DENGVAXIA is not approved for use in individuals younger than 6 years of age. Evidence from clinical studies strongly suggests that DENGVAXIA would be unsafe in individuals younger than 6 years of age because of an increased risk of severe and hospitalized dengue regardless of dengue serostatus [see Warnings and Precautions (5.1)].
11. Dengvaxia Description
DENGVAXIA (Dengue Tetravalent Vaccine, Live) is a sterile suspension for subcutaneous injection. DENGVAXIA is supplied as a vial of lyophilized vaccine antigen, which must be reconstituted at the time of use with 0.6 mL from the accompanying vial of diluent (0.4% sodium chloride). After reconstitution, DENGVAXIA is a clear, colorless suspension (trace amounts of white to translucent proteinaceous particles may be present). [See Dosage and Administration (2.3).]
After reconstitution, each 0.5 mL dose of DENGVAXIA contains 4.5 – 6.0 log10 CCID50 of each of the chimeric yellow fever dengue (CYD) virus serotypes 1, 2, 3, and 4. Each 0.5 mL dose is formulated to contain 2 mg sodium chloride and the following ingredients as stabilizers: 0.56 mg essential amino acids (including L-phenylalanine), 0.2 mg non-essential amino acids, 2.5 mg L-arginine hydrochloride, 18.75 mg sucrose, 13.75 mg D-trehalose dihydrate, 9.38 mg D-sorbitol, 0.18 mg trometamol, and 0.63 mg urea.
Each of the four CYD viruses (CYD-1, CYD-2, CYD-3, and CYD-4) in DENGVAXIA was constructed using recombinant DNA technology by replacing the sequences encoding the pre-membrane (prM) and envelope (E) proteins in the yellow fever (YF) 17D204 vaccine virus genome with those encoding for the homologous sequences of dengue virus serotypes 1, 2, 3, and 4, respectively. Each CYD virus is cultured separately in Vero cells (African Green Monkey kidney) under serum-free conditions, harvested from the supernatant of the Vero cells and purified by membrane chromatography and ultrafiltration. The purified and concentrated harvest of each CYD virus is then diluted in a stabilizer solution to produce the four monovalent drug substances. The final bulk product is a mixture of the four monovalent drug substances diluted in the stabilizer solution. The final bulk product is sterilized by filtration at 0.22 µm, filled into vials and freeze-dried.
DENGVAXIA does not contain preservative.
The vial stoppers for the Lyophilized Vaccine Antigen and Diluent vials of DENGVAXIA are not made with natural rubber latex.
12. Dengvaxia - Clinical Pharmacology
12.1 Mechanism of Action
Following administration, DENGVAXIA elicits dengue-specific immune responses against the four dengue virus serotypes. The exact mechanism of protection has not been determined.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
DENGVAXIA has not been evaluated for carcinogenic or mutagenic potential or impairment of male fertility. Exposure of female rabbits to DENGVAXIA prior to and during gestation did not impair fertility. [See Use in Specific Populations (8.1).]
14. Clinical Studies
14.1 Efficacy
The efficacy of DENGVAXIA was evaluated in two randomized, observer-blind, placebo-controlled, multi-center studies. Study 1 (N=20,869) was conducted in individuals 9 through 16 years of age in four Latin American countries and Puerto Rico; and Study 2 (N=10,275) was conducted in individuals 2 through 14 years of age in five Asia-Pacific countries. A subset of subjects in each study (10% in Study 1; 20% in Study 2) was evaluated for antibodies to dengue virus at the time of enrollment and at later time points. Both studies enrolled subjects irrespective of evidence of previous dengue infection. Subjects were randomized 2:1 to receive either DENGVAXIA or saline placebo and were monitored for symptomatic virologically confirmed dengue (VCD) starting at Day 0. Per protocol vaccine efficacy was assessed beginning 28 days after the third vaccination for 12 months. VCD was defined as an acute febrile illness (temperature ≥38°C on at least 2 consecutive days) virologically confirmed by dengue RT-PCR and/or dengue non-structural protein 1 (NS1) ELISA Antigen Test. For each study, in pre-specified vaccine efficacy analyses including the full age range of subjects enrolled, the pre-specified criterion for demonstrating efficacy of DENGVAXIA against VCD due to any dengue virus serotype and irrespective of previous dengue virus infection, was met (lower bound of 95% CI for vaccine efficacy >25%). These studies were not designed to demonstrate efficacy of DENGVAXIA against individual dengue serotypes.
Given the identification of the increased risk for severe dengue following vaccination with DENGVAXIA and subsequent infection with dengue virus in persons not previously infected with dengue virus [see Adverse Reactions (6.1)], Table 4 presents analyses of vaccine efficacy against VCD due to any dengue virus serotype, limited to subjects who had baseline sera evaluated and who were dengue seropositive at baseline. These analyses include subjects 9 through 16 years of age from Study 1 and subjects 6 through 14 years of age from Study 2.
| DENGVAXIA group Cases (Subjects) | Placebo group Cases (Subjects) | VE % (95% CI)† | |
|---|---|---|---|
|
|||
| Study 1 (Subjects 9 through 16 years of age) | 7 (1034) | 17 (492) | 80.6 (50.7;93.2) |
| Study 2 (Subjects 6 through 14 years of age) | 6 (649) | 15 (337) | 79.5 (44.2;93.5) |
14.2 Immunogenicity of Concomitantly Administered Vaccines
In a randomized, multicenter, open-label study (NCT02992418), 688 subjects aged from 9 through 60 years in the Philippines were randomized in a 1:1 ratio into two groups. In group 1, participants received Adacel concomitantly with the first dose of DENGVAXIA. In group 2, participants received Adacel followed by the first dose of DENGVAXIA one month later. A subgroup analysis was conducted including subjects 9 through 16 years of age who were dengue immune at baseline (Group 1 N=137, Group 2 N=133). There was no evidence of interference in the immune responses to Adacel when concomitantly administered with a first dose of DENGVAXIA as assessed by geometric mean concentrations of antibodies to the pertussis antigens contained in the vaccine and seroprotection rates for diphtheria and tetanus toxoids 28 days after vaccine administration. No information is available on the concommitant administration of Adacel and DENGVAXIA after the three-dose vaccination schedule.
Immune responses following the third dose of DENGVAXIA could not be assessed due to early termination of the study.
16. How is Dengvaxia supplied
16.1 How Supplied
An outer package of 1 dose (NDC 49281-605-01) contains 1 single dose vial of Lyophilized Vaccine Antigen (NDC 49281-606-58) and 1 single dose vial of Saline Diluent (NDC 49281-549-58).
The vial stoppers for the Lyophilized Vaccine Antigen vials and the Saline Diluent vials of DENGVAXIA are not made with natural rubber latex.
16.2 Storage and Handling
Store Lyophilized Vaccine Antigen and Saline Diluent in a refrigerator at 2°C to 8°C (36°F to 46°F). Do not freeze. Protect from light.
Do not use after the expiration date shown on the vial labels of the Lyophilized Vaccine Antigen and Saline Diluent.
After reconstitution, administer DENGVAXIA immediately or store refrigerated at 2°C to 8°C (36°F to 46°F) and use within 30 minutes. Discard reconstituted vaccine if not used within 30 minutes.
17. Patient Counseling Information
Educate vaccine recipients regarding the most common adverse reactions that occur within 14 days following administration of DENGVAXIA (headache, injection site pain, malaise, asthenia, and myalgia).
Inform individuals to seek medical care if they develop signs and symptoms of dengue fever with particular attention to severe dengue warning signs (e.g., high fever, severe abdominal pain or tenderness, persistent vomiting, mucosal bleeding, somnolence and hyperactivity).
Register women who receive DENGVAXIA during pregnancy in the Pregnancy Registry by calling 1-800-822- 2463 (1-800-VACCINE). [See Pregnancy (8.1).]
Instruct vaccine recipients to report adverse reactions to their healthcare provider.
| DENGVAXIA
dengue tetravalent vaccine, live kit |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur VDR | 766693436 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur NVL | 260680254 | MANUFACTURE | |