Drug Detail:Depo-subq provera 104 (Medroxyprogesterone (injection) [ me-drox-ee-proe-jes-ter-one ])
Drug Class: Contraceptives Hormones / antineoplastics Progestins
Highlights of Prescribing Information
DEPO-SUBQ PROVERA 104 (medroxyprogesterone acetate) injectable suspension, for subcutaneous use
Initial U.S. Approval: 1959
WARNING: LOSS OF BONE MINERAL DENSITY
See full prescribing information for complete boxed warning.
- Women who use depo-subQ provera 104 may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible. (5.1)
- It is unknown if use of depo-subQ provera 104 during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life. (5.1)
- Depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate. (1, 5.1)
Indications and Usage for Depo-SubQ Provera 104
Depo-subQ provera 104 is a progestin that is indicated in females of reproductive age for:
- Prevention of pregnancy. (1)
- Management of endometriosis-associated pain. (1)
Limitations of Use:
Use of depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate. (1, 5.1)
Depo-SubQ Provera 104 Dosage and Administration
- Only for healthcare professional administration. (2.1)
- Prior to first injection confirm the patient is not pregnant. (2.1)
- Administer 104 mg of depo-subQ provera 104 by subcutaneous injection into the anterior thigh or abdomen, once every 12 to 14 weeks. (2.1)
- See Full Prescribing Information for recommendations on switching from another contraceptive method to depo-subQ provera 104. (2.2)
- See Full Prescribing Information for important preparation and administration instructions. (2.3)
Dosage Forms and Strengths
Injectable suspension: 104 mg/0.65 mL (3)
Contraindications
- Active thrombophlebitis, or current or history of thromboembolic disorders, or cerebral vascular disease. (4)
- Known, suspected, or past malignancy of the breast. (4)
- Significant liver disease. (4)
- Known hypersensitivity to medroxyprogesterone acetate or any of the ingredients of depo-subQ provera 104. (4)
- Undiagnosed vaginal bleeding. (4)
Warnings and Precautions
- Thromboembolic disorders: Discontinue depo-subQ provera 104 in patients who develop arterial or venous thrombosis. (5.2)
- Breast cancer risks: Monitor women with a family history of breast cancer or a significant risk of breast cancer carefully. (5.3)
- Ectopic pregnancy: Consider ectopic pregnancy if a woman becomes pregnant or complains of severe abdominal pain. (5.4)
- Anaphylaxis: Provide emergency medical treatment. (5.5)
- Injection site reactions (e.g., persistent atrophy, dimpling/indentation, and lump/nodule) have been reported. (5.10)
- Diabetics may be at greater risk of hyperglycemia. (5.12)
- Jaundice and elevated transaminase: Discontinue depo-subQ provera 104 if jaundice or elevated transaminase levels develop. (5.13)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence >5%) are dysfunctional uterine bleeding, headache, increased weight, amenorrhea, and injection site reactions. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Strong CYP3A inhibitors and inducers: Avoid concomitant use. (7)
Use In Specific Populations
- Nursing mothers: Detectable amounts of drug have been identified in the milk of mothers receiving depot-medroxyprogesterone acetate. (8.3)
- Pediatric patients (after menarche): Bone loss is a particular concern. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2020
Full Prescribing Information
WARNING: LOSS OF BONE MINERAL DENSITY
- Women who use depo-subQ provera 104 may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible [see Warnings and Precautions (5.1)].
- It is unknown if use of depo-subQ provera 104 during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk for osteoporotic fracture in later life [see Warnings and Precautions (5.1)].
- Depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate [see Indications and Usage (1) and Warnings and Precautions (5.1)].
1. Indications and Usage for Depo-SubQ Provera 104
Depo-subQ provera 104 is indicated in females of reproductive age for:
- Prevention of pregnancy and
- Management of endometriosis-associated pain.
2. Depo-SubQ Provera 104 Dosage and Administration
2.1 Important Dosage and Administration Instructions
Depo-subQ provera 104 is only for subcutaneous administration and is only to be administered by a healthcare professional.
Use for longer than 2 years is not recommended (unless other birth control methods or medical therapies for endometriosis-associated pain are considered inadequate) due to the impact of long-term depo-subQ provera 104 treatment on bone mineral density (BMD) [see Warnings and Precautions (5.1)].
Prior to the first injection confirm that the patient is not pregnant. For women who are sexually active and who have regular menses, administer the first injection only during the first 5 days of a normal menstrual period. For women who are breast-feeding, administer the first injection during or after the sixth postpartum week.
The recommended dosage of depo-subQ provera 104 is 104 mg given subcutaneously every 12 to 14 weeks. If more than 14 weeks elapse between injections, confirm that the patient is not pregnant before the next injection. Instruct the patient that if they are unable to receive an injection within 12–14 weeks, another contraceptive method should be used until the next depo-subQ provera 104 injection. The dosage does not need to be adjusted for body weight.
Inject the entire contents of the pre-filled syringe using strict aseptic technique into the upper anterior thigh or abdomen, rotating the sites with every injection [see Dosage and Administration (2.3)].
2.2 Switching from Another Method of Contraception
When switching from another contraceptive method to depo-subQ provera 104, administer depo-subQ provera 104 in a manner that ensures continuous contraceptive coverage. Follow the respective recommendations when switching from the contraceptive methods listed below:
- Combined hormonal contraceptives: administer the first injection of depo-subQ provera 104 within 7 days after the last day of using the combined hormonal contraceptive method (i.e., within 7 days after taking the last active pill).
- An implant: administer the first injection of depo-subQ provera 104 on the day of implant removal.
- A contraceptive vaginal ring or transdermal system: administer the first injection of depo-subQ provera 104 on the day the patient would have inserted the next ring or applied the next transdermal system.
- An Intrauterine Device (IUD) or Intrauterine System (IUS): administer the first injection of depo-subQ provera 104 on the day of IUD/IUS removal. If the IUD/IUS is not removed on the first day of the patient's menstrual cycle, instruct patients to use a non-hormonal back-up method of birth control for the first 7 days after administration of depo-subQ provera 104.
- Depot medroxyprogesterone acetate injectable suspension for intramuscular use (DMPA-IM): inject depo-subQ provera 104 12 to 14 weeks after the last dose of DMPA-IM.
2.3 Preparation and Administration Instructions
Prior to injection:
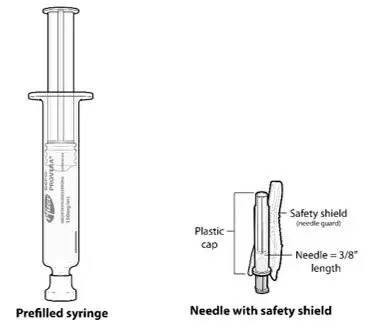
- Ensure all the components in Figure A are available and that depo-subQ provera 104 is at room temperature.
- Shake the pre-filled syringe vigorously prior to injection to ensure appropriate viscosity of the suspension.
- Inspect depo-subQ provera 104 visually for particulate matter and discoloration.
Figure A. Components in the Package
| Step 1: Select & Prepare the Injection Area | |
| Figure B. Preferred injection areas: 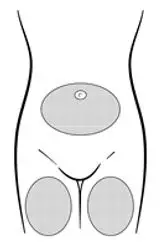 Left or right upper thigh or abdomen |
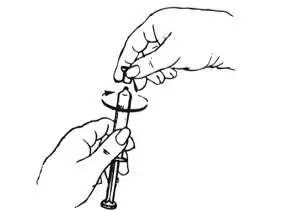
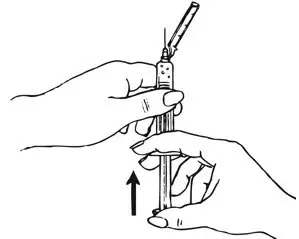
| Step 2: Prepare Syringe | |
| Figure C.
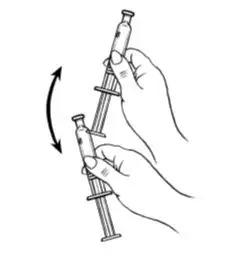 Shake vigorously for 1 minute |
| Figure D.
 |
| Figure E.
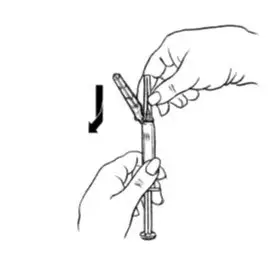 |
| Figure F.
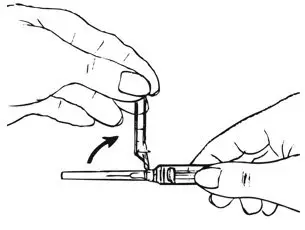 |
| Figure G.
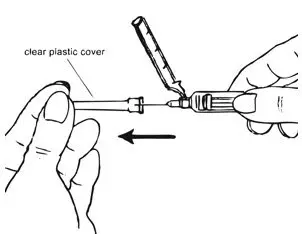 |
| Figure H.
 |
| Step 3: Injecting depo-Sub Q provera 104 | |
| Figure I.
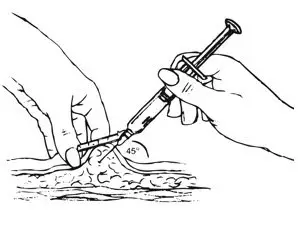 |
Inject slowly until the syringe is empty (Figure J).
| Figure J. Inject slowly (5–7 seconds) 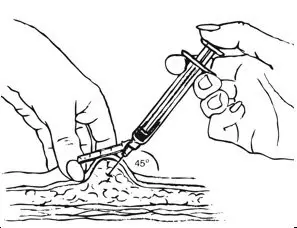 |
| Step 4: Remove the Needle and Activate the Safety Shield | |
| Figure K.
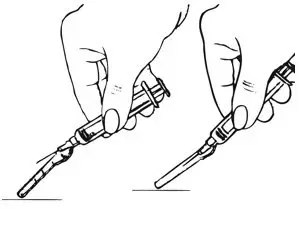 |
| Figure L.
 |
3. Dosage Forms and Strengths
Injectable suspension (104 mg/0.65 mL) in a single-dose pre-filled syringe, packaged with a 26-gauge × 3/8-inch Terumo SurGuard® needle.
4. Contraindications
The use of depo-subQ provera 104 is contraindicated in the following conditions:
- Active thrombophlebitis, or current or history of thromboembolic disorders, or cerebral vascular disease [see Warnings and Precautions (5.2)].
- Known, suspected, or past malignancy of the breast [see Warnings and Precautions (5.3)].
- Significant liver disease [see Warnings and Precautions (5.13)].
- Known hypersensitivity to medroxyprogesterone acetate or any of the ingredients in depo-subQ provera 104 [see Warnings and Precautions (5.5)].
- Undiagnosed vaginal bleeding [see Warnings and Precautions (5.11)].
5. Warnings and Precautions
5.1 Loss of Bone Mineral Density
Use of depo-subQ provera 104 reduces serum estrogen levels and is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of depo-subQ provera 104 by younger women will reduce peak bone mass and increase the risk for osteoporotic fracture in later life.
A study to assess the reversibility of loss of BMD in adolescents was conducted with DMPA-IM. After discontinuing DMPA-IM in these adolescents, mean BMD loss at the total hip and femoral neck did not fully recover by 5 years (60 months) post-treatment in the sub-group of adolescents who were treated for more than 2 years [see Clinical Studies (14.4)]. Similarly, in adults, there was only partial recovery of mean BMD at the total hip, femoral neck, and lumbar spine towards baseline by 2 years post-treatment [see Clinical Studies (14.3)].
The use of depo-subQ provera 104 is not recommended as a long-term (i.e., longer than 2 years) birth control method or medical therapy for endometriosis-associated pain unless other options are considered inadequate. BMD should be evaluated when a woman needs to continue to use depo-subQ provera 104 long-term. In adolescents, interpretation of BMD results should take into account patient age and skeletal maturity.
Other birth control methods or therapies for endometriosis-associated pain should be considered in the risk/benefit analysis for the use of depo-subQ provera 104 in women with osteoporosis risk factors. Depo-subQ provera 104 can pose an additional risk in patients with risk factors for osteoporosis (e.g., metabolic bone disease, chronic alcohol and/or tobacco use, anorexia nervosa, strong family history of osteoporosis, or chronic use of drugs that can reduce bone mass such as anticonvulsants or corticosteroids).
5.2 Arterial and Venous Thromboembolic Disorders
There have been reports of serious arterial and venous thrombotic events in women treated with DMPA-IM. Women with a history of thromboembolic disorders were not studied in clinical trials of depo-subQ provera 104. Although no causal relationship between the use of depo-subQ provera 104 and thrombotic events has been clearly established, patients who develop arterial or venous thrombosis while taking depo-subQ provera 104 should discontinue treatment.
Do not re-administer depo-subQ provera 104 pending examination if there is a sudden onset of a suspected vascular ocular event (e.g., partial or complete loss of vision, proptosis, or diplopia) or migraine. Do not re-administer depo-subQ provera 104 if examination reveals papilledema or retinal vascular lesions.
5.4 Ectopic Pregnancy
Healthcare professionals should be alert to the possibility of an ectopic pregnancy among women using depo-subQ provera 104 who become pregnant or complain of severe abdominal pain.
5.5 Anaphylaxis
Serious anaphylactic reactions have been reported in women using depo-subQ provera 104. If an anaphylactic reaction occurs, appropriate emergency medical treatment should be administered.
5.6 Fluid Retention
Because progestational drugs including depo-subQ provera 104 may cause fluid retention, monitor patients with conditions that might be affected by fluid retention.
5.7 Weight Gain
Weight gain is a common occurrence in women using depo-subQ provera 104. In three large clinical trials using depo-subQ provera 104, the mean weight gain was 3.5 lb (1.6 kg) in the first year of use. In a small, two-year study comparing depo-subQ provera 104 to DMPA-IM, the mean weight gain observed for women using depo-subQ provera 104 [7.5 lb (3.4 kg)] was similar to the mean weight gain for women using DMPA-IM [7.6 lb (3.5 kg)].
Although there are no data related to weight gain beyond 2 years for depo-subQ provera 104, the data on DMPA-IM may be relevant. In a clinical study, after five years, 41 women using Depo-Provera CI (150 mg) had a mean weight gain of 11.2 lb (5.1 kg), while 114 women using non-hormonal contraception had a mean weight gain of 6.4 lb (2.9 kg).
5.8 Delayed Return of Ovulation or Fertility
Return to ovulation is likely to be delayed after stopping depo-subQ provera 104, as demonstrated in a study of 15 women who received multiple doses of depo-subQ provera 104:
- Median time to ovulation was 10 months after the last injection.
- Earliest return to ovulation was 6 months after the last injection.
- 12 women (80%) ovulated within 1 year of the last injection.
However, ovulation has occurred as early as 14 weeks after a single dose of depo-subQ provera 104; therefore, administer the next depo-subQ provera 104 12 to 14 weeks after the last injection.
Return to fertility also is likely to be delayed after stopping therapy. Among 28 women using depo-subQ provera 104 for contraception who stopped treatment to become pregnant, 7 women were lost to follow-up. One woman became pregnant within one year of her last injection and another woman became pregnant 443 days after her last injection. The remaining 19 women had not become pregnant; it is not known if these 19 women were still attempting to become pregnant or if they had started a new contraceptive method.
5.9 Depression
Depression (3% of depo-subQ provera 104-treated patients) and other mood disorders have been reported in clinical trials of depo-subQ provera 104 [see Adverse Reactions (6.1)]. Patients with a history of depression or who are on treatment for depression may be at increased risk for depression recurrence or exacerbation and for associated mood disorders while receiving depo-subQ provera 104. Therefore, patients should be monitored for symptoms of depression and mood changes.
5.10 Injection Site Reactions
In five clinical studies of depo-subQ provera 104 involving 2325 women (282 treated for up to 6 months, 1780 treated for up to 1 year, and 263 women treated for up to 2 years), 5% of women reported injection site reactions, and 1% had persistent skin changes (small areas of induration or atrophy).
In post-marketing experience, injection site reactions such as persistent atrophy of the injection site, dimpling/indentation, and injection site lump/nodule have been reported.
5.11 Bleeding Irregularities
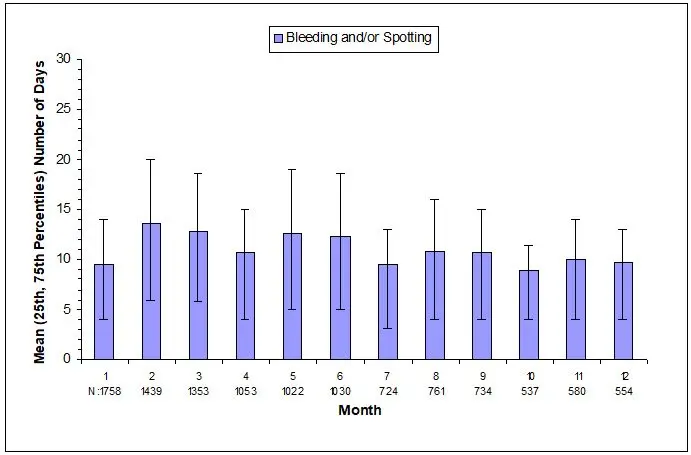
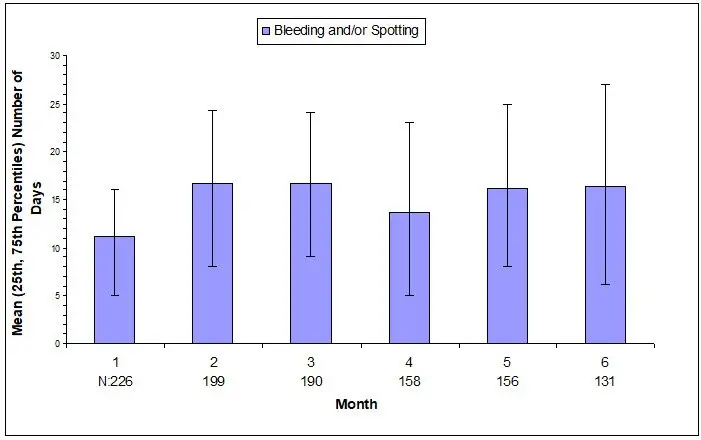
Most women using depo-subQ provera 104 experienced changes in menstrual bleeding patterns, such as amenorrhea, irregular unpredictable spotting or bleeding, prolonged spotting or bleeding, or heavy bleeding [see Adverse Reactions (6.1)]. Fewer women experienced irregular bleeding and more experienced amenorrhea with longer term use of depo-subQ provera 104, consistent with expected endometrial thinning effects.
In three contraception trials, 39% of 2053 depo-subQ provera 104-treated women experienced amenorrhea during Month 6, and 57% experienced amenorrhea during Month 12. In two endometriosis trials using depo-subQ provera 104, 24% of 289 women experienced amenorrhea during Month 6 [see Adverse Reactions (6.1)].
If abnormal bleeding is persistent or severe, evaluate the patient for underlying pathology or pregnancy.
5.12 Risk of Hyperglycemia in Patients with Diabetes
Some patients receiving progestins may exhibit a decrease in glucose tolerance; therefore, patients with diabetes may be at greater risk of hyperglycemia.
5.13 Jaundice and Elevated Transaminase
Discontinue depo-subQ provera 104 if jaundice or elevated transaminase levels develop. Depo-subQ provera 104 may be resumed after both the jaundice and elevated transaminase levels resolve, and the healthcare professional determines that depo-subQ provera 104 did not cause the abnormalities.
6. Adverse Reactions/Side Effects
The following important adverse reactions are described in more detail in other sections of the prescribing information:
- Loss of bone mineral density [see Warnings and Precautions (5.1)]
- Arterial and venous thromboembolic disorders [see Warnings and Precautions (5.2)]
- Anaphylaxis [see Warnings and Precautions (5.5)]
- Fluid retention [see Warnings and Precautions (5.6)]
- Delayed return of ovulation or fertility [see Warnings and Precautions (5.8)]
- Depression [see Warnings and Precautions (5.9)]
- Injection site reactions [see Warnings and Precautions (5.10)]
- Bleeding irregularities [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to depo-subQ provera 104 in five clinical trials involving 2325 women including 2043 women who received treatment for contraception (1780 treated up to 1 year and 263 treated for up to 2 years) and 282 women for endometriosis for up to 6 months. In these pooled trials, 9% of women discontinued treatment due to an adverse reaction and the most common reason for discontinuation was dysfunctional uterine bleeding (3%).
Adverse Reactions in the Contraception Adult Studies
Table 1 presents frequently reported adverse reactions (>1%) in the contraception pooled studies. In these studies, the most frequently reported adverse reactions (>5%) were dysfunctional uterine bleeding (e.g., irregular, increased, decreased, or spotting), headache, increased weight, amenorrhea, and injection site reactions (e.g., pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling or lipodystrophy).
The frequency reported is based on the all-causality incidence in the pooled results of the three contraception studies. Closely related "Adverse Reaction" terms were grouped but individual patients reporting two or more grouped events were only counted once.
| Adverse Reaction | Frequency |
|---|---|
| Dysfunctional uterine bleeding (irregular, increase, decrease, spotting) | 18% |
| Headache | 9% |
| Increased weight (see below) | 7% |
| Amenorrhea | 6% |
| Injection site reactions (such as pain/tenderness, nodule/lump, persistent atrophy/indentation/dimpling, lipodystrophy) | 6% |
| Vaginitis, including candidiasis and bacterial | 5% |
| Abdominal pain | 4% |
| Urinary tract infections | 4% |
| Acne | 4% |
| Depression | 3% |
| Decreased libido | 3% |
| Nausea | 3% |
| Back pain | 3% |
| Breast pain/tenderness | 2% |
| Fatigue | 2% |
| Anxiety | 1% |
| Irritability | 1% |
| Dizziness | 1% |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of DMPA-IM. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Immune system disorders: anaphylactic reaction, anaphylactoid reaction, angioedema
- Vascular disorders: pulmonary embolism, deep vein thrombosis, thrombophlebitis
- Musculoskeletal and connective tissue disorders: osteoporosis (including osteoporotic fractures)
- Reproductive system and breast disorders: prolonged anovulation, unexpected pregnancy, uterine hyperplasia
- Respiratory, thoracic and mediastinal disorders: hoarseness
- Skin and subcutaneous tissue disorders: increased body odor
- Gastrointestinal disorders: gastrointestinal disturbances
- General disorders and administration site conditions: axillary swelling, chills, thirst
8. Use In Specific Populations
8.1 Pregnancy
There is no use for depo-subQ provera 104 in pregnancy and therefore depo-subQ provera 104 should be discontinued during pregnancy. There appears to be little or no increased risk of birth defects in women who have inadvertently been exposed to medroxyprogesterone acetate injections in early pregnancy. Neonates exposed to medroxyprogesterone acetate in-utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual, or social development.
8.3 Nursing Mothers
Although medroxyprogesterone acetate is detectable in the milk of mothers receiving DMPA-IM, milk composition, quality, and amount do not appear to be adversely affected. Effects on milk production and lactation initiation/duration remain unclear when administered before 6 weeks after delivery. Neonates and infants exposed to medroxyprogesterone acetate from breast milk have been studied for developmental and behavioral effects through puberty, and no adverse effects have been noted.
8.4 Pediatric Use
Depo-subQ provera 104 is indicated for the prevention of pregnancy and management of endometriosis-associated pain in females of reproductive age. Efficacy is expected to be the same for post-menarchal females under the age of 17 as for users 17 years and older.
Use of depo-subQ provera 104 is associated with significant loss of bone mineral density (BMD). This loss of BMD is of particular concern during adolescence, a critical period of bone accretion. It is unknown if use of depo-subQ provera 104 by female adolescents will reduce peak bone mass and increase the risk for osteoporotic fractures in later life. In a study of adolescent females (12–18 years of age) receiving DMPA-IM for contraception, mean BMD 2 years after starting DMPA-IM decreased 1.9% (spine), 4.3% (total hip), and 4.2% (femoral neck). In those adolescents who used DMPA-IM for more than 2 years, mean BMD at total hip and femoral neck did not return to baseline within 5 years.
Depo-subQ provera 104 is not indicated before menarche.
11. Depo-SubQ Provera 104 Description
Depo-subQ provera 104 contains medroxyprogesterone acetate (MPA), a derivative of progesterone, as its active ingredient. MPA is a white to off-white, odorless crystalline powder that is stable in air and that melts between 205°C and 209°C. It is freely soluble in chloroform, soluble in acetone and dioxane, sparingly soluble in alcohol and methanol, slightly soluble in ether, and insoluble in water.
The chemical name for MPA is 17-hydroxy-6α-methylpregn-4-ene-3,20-dione 17-acetate. The structural formula is as follows:
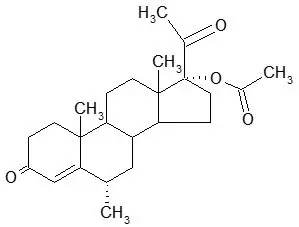
Depo-subQ provera 104 for subcutaneous use is available in pre-filled syringes, each containing 0.65 mL (104 mg) of sterile medroxyprogesterone acetate injectable suspension.
Each 0.65 mL contains the following inactive ingredients:
| Methylparaben | 1.040 mg |
| Propylparaben | 0.098 mg |
| Sodium Chloride | 5.200 mg |
| Polyethylene Glycol | 18.688 mg |
| Polysorbate 80 | 1.950 mg |
| Monobasic Sodium Phosphate H2O | 0.451 mg |
| Dibasic Sodium Phosphate 12H2O | 0.382 mg |
| Methionine | 0.975 mg |
| Povidone | 3.250 mg |
| Water for Injection | qs |
When necessary, the pH is adjusted with sodium hydroxide or hydrochloric acid, or both.
12. Depo-SubQ Provera 104 - Clinical Pharmacology
12.1 Mechanism of Action
Depo-subQ provera 104 inhibits the secretion of gonadotropins, which primarily prevents follicular maturation and ovulation and causes thickening of cervical mucus. These actions contribute to its contraceptive effect.
Suppression of serum estradiol concentrations is likely to be responsible for the therapeutic effect on endometriosis-associated pain.
12.2 Pharmacodynamics
The following laboratory tests are expected to be affected by progestins including depo-subQ provera 104:
- Plasma and urinary steroid levels are decreased (e.g., progesterone, estradiol, pregnanediol, testosterone, cortisol).
- Gonadotropin levels are decreased.
- Sex-hormone-binding-globulin concentrations are decreased.
- Histology specimens may demonstrate changes consistent with progestin effects.
The following laboratory tests may be affected by depo-subQ provera 104, however the clinical significance is unknown:
- Protein-bound iodine and butanol extractable protein-bound iodine may increase.
- T3-uptake values may decrease.
- Coagulation test values for prothrombin (Factor II), and Factors VII, VIII, IX, and X may increase.
- Sulfobromophthalein and other liver function test values may be increased.
- The effects of medroxyprogesterone acetate on lipid metabolism are inconsistent. Both increases and decreases in total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol have been observed in studies.
12.3 Pharmacokinetics
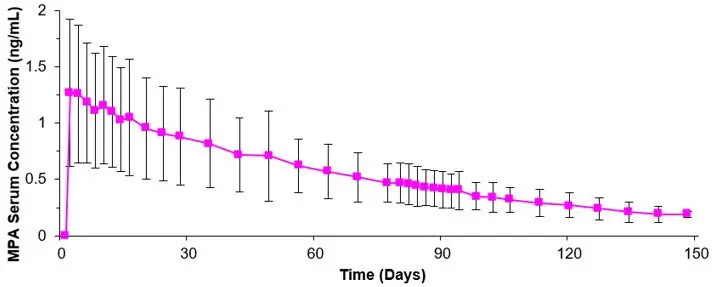
The pharmacokinetic parameters of MPA following a single subcutaneous injection of depo-subQ provera 104 in healthy women (n=42) are shown in Table 2 and Figure P.
| Cmax
(ng/mL) | Tmax
(day) | C91
(ng/mL) | AUC0–91
(ng∙day/mL) | AUC0–∞
(ng∙day/mL) | t½ (day) |
|
|---|---|---|---|---|---|---|
| Abbreviations: Cmax=peak serum concentration; Tmax=time when Cmax is observed; C91=serum concentration at 91 days; AUC0–91 and AUC0–∞=area under the concentration-time curve over 91 days or infinity, respectively; t½=terminal half-life. | ||||||
| Mean | 1.56 | 8.8 | 0.402 | 66.98 | 92.84 | 43 |
| Min | 0.53 | 2.0 | 0.133 | 20.63 | 31.36 | 16 |
| Max | 3.08 | 80.0 | 0.733 | 139.79 | 162.29 | 114 |
Following subcutaneous administration of single depo-subQ provera 104 doses ranging from 50 to 150 mg (0.48 and 1.4 times the recommended dose, respectively), the AUC and Cmin (Day 91) increased with higher doses, but there was considerable overlap across dose levels. Serum MPA concentrations at Day 91 increased in a dose proportional manner but Cmax did not appear to increase proportionally with increasing dose. The AUC data were suggestive of dose linearity.
14. Clinical Studies
14.1 Contraception Studies
In three open label clinical studies, depo-SubQ provera 104 (104 mg given every three months subcutaneously), was administered to healthy, sexually-active, nonpregnant women 18 to 49 years of age who desired long-term contraception. In these three studies, no pregnancies were detected among 2042 women treated with depo-subQ provera 104 for up to 1 year. In women less than 36 years of age (at baseline), the Pearl Index pregnancy rate in cycles in which no other contraceptive methods were used, was 0 pregnancies per 100 women-years of use (upper 95% CI = 0.25).
14.2 Endometriosis Studies
The efficacy of depo-subQ provera 104 in the reduction of endometriosis-associated pain in women with the signs and symptoms of endometriosis was demonstrated in two active comparator-controlled studies in pre-menopausal women 18 to 49 years of age with laparoscopically diagnosed endometriosis and persistent endometriosis pain symptoms (i.e., Studies 268 and 270). Each study assessed endometriosis-associated pain over 6 months of treatment and recurrence of symptoms for 12-months post treatment.
Subjects were treated for six months with depo-subQ provera 104 [104 mg given subcutaneously every 3 months (2 injections)] or leuprolide [11.25 mg given subcutaneously every 3 months (2 injections) or 3.75 mg given subcutaneously every month (6 injections)]. Study 268 was conducted in the U.S. and Canada and enrolled 274 subjects (136 subjects received depo-subQ provera 104 and 138 subjects received leuprolide). Study 270 was conducted in South America, Europe, and Asia, and enrolled 299 subjects (153 subjects received depo-subQ provera 104 and 146 subjects received leuprolide).
Reduction in endometriosis pain was evaluated using a modified Biberoglu and Behrman scale that consisted of three patient-reported symptoms (i.e., dysmenorrhea, dyspareunia, and pelvic pain not related to menses) and two signs assessed during pelvic examination (i.e., pelvic tenderness and induration). For each category, a favorable response was defined as improvement of at least 1 unit (severity was assessed on a scale of 0 to 3) relative to baseline score (Figure Q).
| Favorable Response = reduction in severity of symptom or sign of ≥1 point on a scale of 0 to 3, as compared to baseline. |
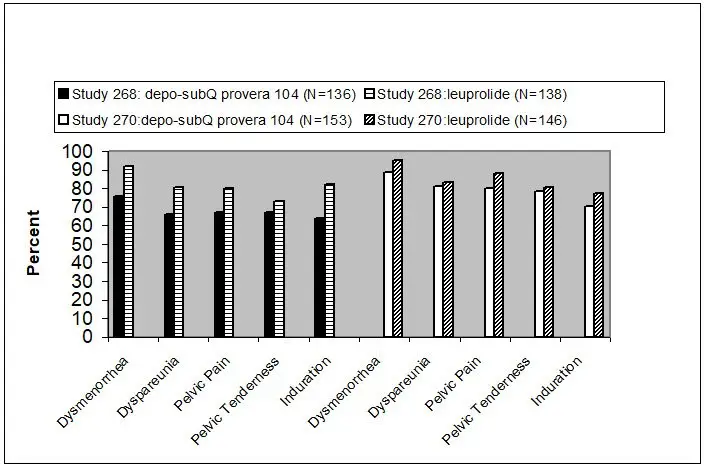 |
Additionally, scores from each of the five categories were combined into a composite score that was considered a global measurement of overall disease improvement. For subjects with baseline scores for each of the 5 categories, a mean decrease of 4 points relative to baseline was considered a clinically meaningful improvement. Across both studies, the mean changes in the composite score met the protocol-defined criterion for improvement for the depo-subQ provera 104 and leuprolide treatment groups.
In the clinical trials, treatment with depo-subQ provera 104 was limited to six months. Data on the persistence of benefit with longer treatment are not available.
14.3 Bone Mineral Density in Women Treated with Depo-medroxyprogesterone acetate for Contraception
In a study that compared changes in bone mineral density (BMD) in adult women using depo-subQ provera 104 or DMPA-IM for contraception, both treatments showed BMD reductions in the lumbar spine, total hip, and femoral neck. Mean percent changes in BMD in depo-subQ provera 104-treated women are shown in Table 3.
| Time on Treatment | Lumbar Spine | Total Hip | Femoral Neck |
|---|---|---|---|
| Mean % Change (95% CI) | Mean % Change (95% CI) | Mean % Change (95% CI) |
|
| 1 year (n=166) | -2.7 (-3.1 to -2.3) | -1.7 (-2.1 to -1.3) | -1.9 (-2.5 to -1.4) |
| 2 years (n=106) | - 4.1 (-4.6 to -3.5) | -3.5 (-4.2 to -2.7) | -3.5 (-4.3 to -2.6) |
14.4 Bone Mineral Density Changes in Adolescent Females (12 to 18 years of age) Treated with DMPA-IM
The effect of DMPA-IM on BMD in adolescents is described below, and the effect of depo-subQ provera 104 on BMD in adolescents is expected to be similar. The impact of DMPA-IM use for up to 240 weeks (4.6 years) was evaluated in an open-label non-randomized clinical study in 389 adolescent females (12 to 18 years of age). Use of DMPA-IM was associated with a significant decline from baseline in BMD.
Partway through the trial, DMPA-IM administration was stopped (at 120 weeks). The mean number of injections per DMPA-IM user was 9.3. Table 5 summarizes the study findings. The decline in BMD at total hip and femoral neck was greater with longer duration of use. The mean decrease in BMD at 240 weeks was more pronounced at total hip (-6.4%) and femoral neck (-5.4%) compared to lumbar spine (-2.1%).
Adolescents in the untreated cohort had an increase in BMD during the period of growth following menarche. However, the two cohorts were not matched at baseline for age, gynecologic age, race, BMD, and other factors that influence the rate of acquisition of BMD.
| Duration of Treatment | DMPA-IM (150 mg) | Unmatched, Untreated Cohort | ||
|---|---|---|---|---|
| N | Mean % Change | N | Mean % Change | |
| Total Hip BMD | ||||
| Week 60 (1.2 years) | 113 | -2.75 | 166 | 1.22 |
| Week 120 (2.3 years) | 73 | -5.40 | 109 | 2.19 |
| Week 240 (4.6 years) | 28 | -6.40 | 84 | 1.71 |
| Femoral Neck BMD | ||||
| Week 60 | 113 | -2.96 | 166 | 1.75 |
| Week 120 | 73 | -5.30 | 108 | 2.83 |
| Week 240 | 28 | -5.40 | 84 | 1.94 |
| Lumbar Spine BMD | ||||
| Week 60 | 114 | -2.47 | 167 | 3.39 |
| Week 120 | 73 | -2.74 | 109 | 5.28 |
| Week 240 | 27 | -2.11 | 84 | 6.40 |
14.5 Bone Fracture Incidence in Women Treated with Depo-medroxyprogesterone acetate for Contraception
A retrospective cohort study to assess the association between DMPA-IM injection and the incidence of bone fractures was conducted in 312,395 female contraceptive users in the UK. The incidence rates of fracture were compared between DMPA-IM users and contraceptive users who had no recorded use of DMPA-IM. The Incident Rate Ratio (IRR) for any fracture during the follow-up period (mean=5.5 years) was 1.41 (95% CI 1.35, 1.47). It is not known if this is due to DMPA-IM use or to other related lifestyle factors that have a bearing on fracture rate.
In the study, when cumulative exposure to DMPA-IM was calculated, the fracture rate in users who received fewer than 8 injections was higher than that in women who received 8 or more injections. However, it is not clear that cumulative exposure, which may include periods of intermittent use separated by periods of non-use, is a useful measure of risk, as compared to exposure measures based on continuous use.
There were very few osteoporotic fractures (fracture sites known to be related to low BMD) in the study overall, and the incidence of osteoporotic fractures was not found to be higher in DMPA-IM users compared to non-users.
Importantly, this study could not determine whether use of DMPA-IM has an effect on fracture rate later in life. Given the similar effects on BMD from depo-subQ provera 104 and DMPA-IM described above, bone fracture incidence may also be expected to be similar.
14.6 Bone Mineral Density in Women Treated with Depo-subQ provera 104 for Endometriosis
In two clinical studies of 573 adult women with endometriosis, the BMD effects of 6 months of depo-subQ provera 104 treatment (104 mg subcutaneously every 3 months) were compared to 6 months of leuprolide treatment (either 11.25 mg given subcutaneously every 3 months or 3.75 mg given subcutaneously every month). Subjects were then observed after treatment completion, for an additional 12 months. See Table 7 for the results.
| Time of BMD Measurement | Lumbar Spine | Total Hip | ||||||
|---|---|---|---|---|---|---|---|---|
| depo-subQ provera 104 | Leuprolide | depo-subQ provera 104 | Leuprolide | |||||
| N | Mean % Change | N | Mean % Change | N | Mean % Change | N | Mean % Change | |
| Month 6 of treatment (End of Treatment) | 208 | -1.20 | 229 | -4.10 | 207 | -0.03 | 227 | -1.83 |
| 6 months post-treatment | 168 | -1.06 | 180 | -2.75 | 169 | -0.05 | 181 | -1.59 |
| 12 months post-treatment | 124 | -0.54 | 133 | -1.48 | 125 | 0.39 | 134 | -1.15 |
15. References
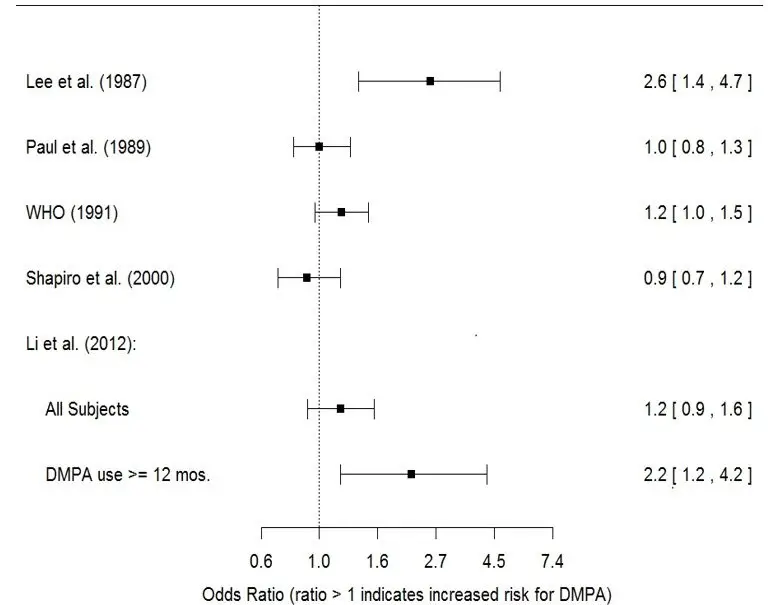
- Li CI, Beaber EF, Tang MCT et al. Effect of Depo-Medroxyprogesterone Acetate on Breast Cancer Risk among Women 20 to 44 years of Age. Cancer Research 2012; 72:2028-2035.
- Paul C, Skegg DCG, Spears GFS. Depot medroxyprogesterone (Depo-Provera) and risk of breast cancer. Br Med J 1989; 299:759-62.
16. How is Depo-SubQ Provera 104 supplied
| DEPO-SUBQ PROVERA
medroxyprogesterone acetate injection, suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | ANALYSIS(0009-4709) , MANUFACTURE(0009-4709) , PACK(0009-4709) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-4709) , API MANUFACTURE(0009-4709) , PACK(0009-4709) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterigenics Belgium Petit-Rechain S.A. | 370026481 | ANALYSIS(0009-4709) | |