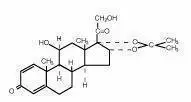
Drug Detail:Desowen (Desonide topical [ des-oh-nide ])
Drug Class: Topical steroids
DesOwen - Clinical Pharmacology
Like other topical corticosteroids, desonide has anti-inflammatory, antipruritic and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Pharmacokinetics:
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours have not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.
Studies performed with DesOwen® (desonide cream and lotion) Cream and Lotion indicate that they are in the low to medium range of potency as compared with other topical corticosteroids.
Precautions
Pediatric use:
Safety and effectiveness in pediatric patients have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression when they are treated with topical corticosteroids. They are therefore also at greater risk of glucocorticosteroid insufficiency after withdrawal of treatment and of Cushing’s syndrome while on treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
PACKAGE LABEL - Desowen Cream
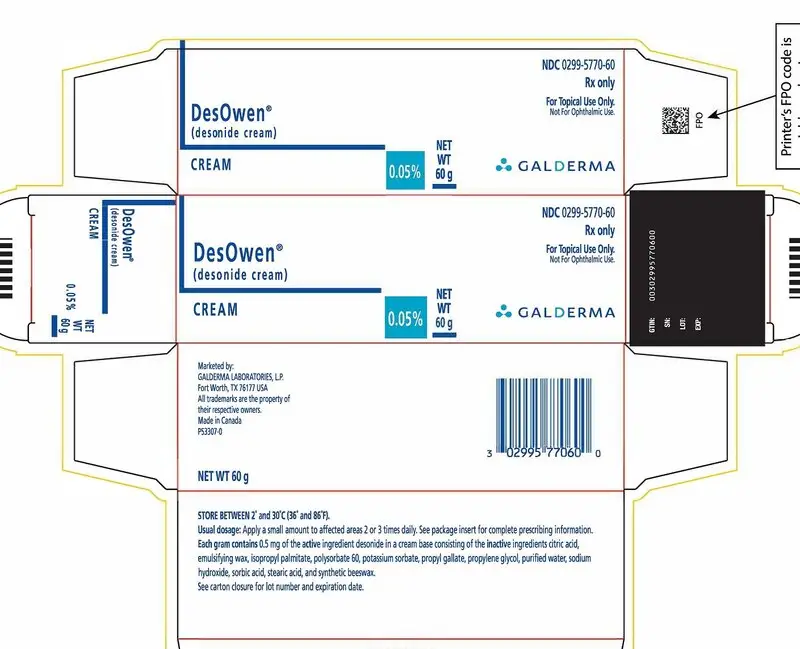
NDC 0299-5770-60
Rx Only
For Topical Use Only.
Not for Ophthalmic Use.
DesOwen®
(desonide cream)
Cream 0.05% NET WT. 60 g
GALDERMA
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
All trademarks are the property of their respective owners.
Made in Canada
P53307-0
Store between 2° and 30°C (35° and 86°F).
Usual dosage: Apply a small amount to affected areas 2 or 3 times daily. See package insert for complete prescribing information.
Each gram contains 0.5 mg of the active ingredient desonide in a cream base consisting of the inactive ingredients citric acid, emulsifying wax, isopropyl palmitate, polysorbate 60, potassium sorbate, propyl gallate, propylene glycol, purified water, sodium hydroxide, sorbic acid, stearic acid and synthetic beeswax.
See carton closure for lot number and expiration date.
PACKAGE Label - Desown Lotion
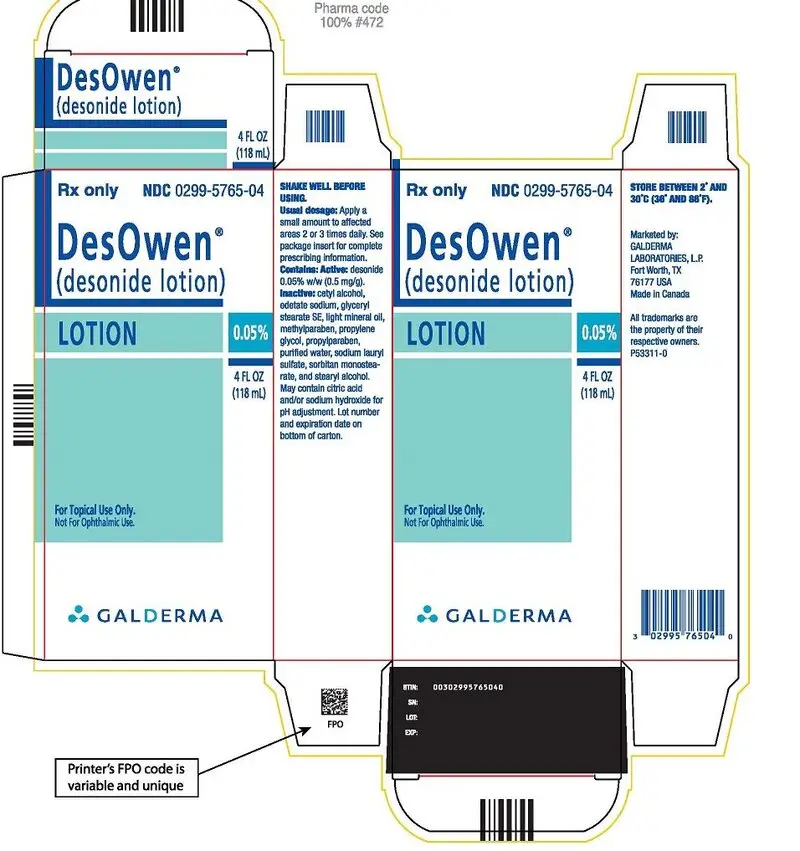
Rx Only NDC 0299-5765-04
DesOwen®
(desonide lotion)
LOTION 0.05%
4 FL OZ
(118 mL)
For Topical Use Only.
Not for ophthalmic Use
GALDERMA
SHAKE WELL BEFORE USING.
Usual dosage: Apply a small amount to affected areas 2 or 3 times daily. See package insert for complete prescribing information.
Contains: Active: desonide 0.05% w/w (0.5 mg/g). Inactive: cetyl alcohol, edetate sodium, glyceryl stearate SE, light mineral oil, methylparaben, propylene glycol, propylparaben, purified water, sodium lauryl sulfate, sorbitan monostearate, and stearyl alcohol. May contain citric acid and/or sodium hydroxide for pH adjustment. Lot number and expiration date on bottom of carton.
STORE BETWEEN 2° AND 30° C (36° AND 86°F)
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas
76177 USA
Made in Canada.
All trademarks are the property of their respective owners.
P53311-0
| DESOWEN
desonide cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| DESOWEN
desonide lotion |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224526 | MANUFACTURE(0299-5770, 0299-5765) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| G Production Inc. | 251676961 | manufacture(0299-5765, 0299-5770) | |