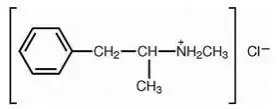
Drug Detail:Desoxyn (Methamphetamine [ meth-am-fet-a-meen ])
Drug Class: Anorexiants CNS stimulants
Contraindications
In patients known to be hypersensitive to amphetamine, or other components of DESOXYN. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other amphetamine products (see ADVERSE REACTIONS).
Patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of stopping MAOIs (including MAOIs such as linezolid or intravenous methylene blue), because of an increased risk of hypertensive crisis (see WARNINGS and DRUG INTERACTIONS). It is also contraindicated in patients with glaucoma, advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism or known hypersensitivity or idiosyncrasy to sympathomimetic amines. Methamphetamine should not be given to patients who are in an agitated state or who have a history of drug abuse.
Precautions
Drug Interactions:
Insulin requirements in diabetes mellitus may be altered in association with the use of methamphetamine and the concomitant dietary regimen.
Methamphetamine may decrease the hypotensive effect of guanethidine.
DESOXYN should not be used concurrently with monoamine oxidase inhibitors (see CONTRAINDICATIONS).
Concurrent administration of tricyclic antidepressants and indirect-acting sympathomimetic amines such as the amphetamines, should be closely supervised and dosage carefully adjusted.
Phenothiazines are reported in the literature to antagonize the CNS stimulant action of the amphetamines.
Adverse Reactions/Side Effects
The following are adverse reactions in decreasing order of severity within each category that have been reported:
Cardiovascular: Elevation of blood pressure, tachycardia and palpitation. Fatal cardiorespiratory arrest has been reported, mostly in the context of abuse/misuse.
Central Nervous System: Psychotic episodes have been rarely reported at recommended doses. Dizziness, dysphoria, overstimulation, euphoria, insomnia, tremor, restlessness and headache. Exacerbation of motor and phonic tics and Tourette’s syndrome.
Gastrointestinal: Diarrhea, constipation, dryness of mouth, unpleasant taste and other gastrointestinal disturbances.
Hypersensitivity: Urticaria.
Endocrine: Impotence and changes in libido; frequent or prolonged erections.
Musculoskeletal: Rhabdomyolysis.
Miscellaneous: Suppression of growth has been reported with the long-term use of stimulants in children (see WARNINGS).
Skin and Subcutaneous Tissue Disorders: Alopecia.
To report SUSPECTED ADVERSE REACTIONS, contact Key Therapeutics, LLC. at 1-888-981-8337 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Serotonin syndrome has also been reported. Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
| DESOXYN
methamphetamine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Key Therapeutics (080318791) |