Drug Detail:Dipentum (Olsalazine [ ole-sal-a-zeen ])
Drug Class: 5-aminosalicylates
Dipentum Description
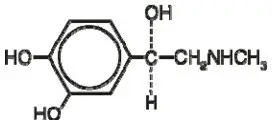
The active ingredient in DIPENTUM Capsules (olsalazine sodium) is the sodium salt of a salicylate, disodium 3,3'-azobis (6-hydroxybenzoate) a compound that is effectively bioconverted to mesalamine (5-aminosalicylic acid,5-ASA), an aminosalicylate. Its empirical formula is C14H8N2Na2O6 with a molecular weight of 346.21.
The structural formula is:
Olsalazine sodium is a yellow crystalline powder, which melts with decomposition at 240°C. It is the sodium salt of a weak acid, soluble in water and DMSO, and practically insoluble in ethanol, chloroform, and ether. Olsalazine sodium has acceptable stability under acidic or basic conditions.
DIPENTUM is supplied in hard gelatin capsules for oral administration. The inert ingredient in each 250 mg capsule of olsalazine sodium is magnesium stearate. The capsule shell contains the following inactive ingredients: black iron oxide, caramel, gelatin, and titanium dioxide.
Dipentum - Clinical Pharmacology
After oral administration, olsalazine has limited systemic bioavailability. Based on oral and intravenous dosing studies, approximately 2.4% of a single 1.0 g oral dose is absorbed. Less than 1% of olsalazine is recovered in the urine. The remaining 98 to 99% of an oral dose will reach the colon, where each molecule is rapidly converted into two molecules of 5-aminosalicylic acid (5-ASA) by colonic bacteria and the low prevailing redox potential found in this environment. The liberated 5-ASA is absorbed slowly resulting in very high local concentrations in the colon.
The conversion of olsalazine to mesalamine (5-ASA) in the colon is similar to that of sulfasalazine, which is converted into sulfapyridine and mesalamine. The usual dose of sulfasalazine for maintenance of remission in patients with ulcerative colitis is 2 grams daily, which would provide approximately 0.8 gram of mesalamine to the colon. More than 0.9 gram of mesalamine would usually be made available in the colon from 1 gram of olsalazine.
The mechanism of action of mesalamine (and sulfasalazine) is not fully understood, but appears to be a topical anti-inflammatory effect on colonic epithelial cells. Mucosal production of arachidonic acid (AA) metabolites, both through the cyclooxygenase pathways (i.e., prostanoids) and through the lipoxygenase pathways (i.e., leukotrienes [LTs] and hydroxyeicosatetraenoic acids [HETEs]) is increased in patients with ulcerative colitis, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin (PG) production in the colon.
Pharmacokinetics
The pharmacokinetics of olsalazine are similar in both healthy volunteers and in patients with ulcerative colitis. Maximum serum concentrations of olsalazine appear after approximately 1 hour and, even after a 1.0 g single dose, are low (e.g., 1.6 to 6.2 µmol/L). Olsalazine has a very short serum half-life, approximately 0.9 hour. Olsalazine is more than 99% bound to plasma proteins. It does not interfere with protein binding of warfarin. The urinary recovery of olsalazine is below 1%. Total recovery of oral 14C-labeled olsalazine in animals and humans ranges from 90 to 97%. Approximately 0.1% of an oral dose of olsalazine is metabolized in the liver to olsalazine-O-sulfate (olsalazine-S). Olsalazine-S, in contrast to olsalazine has a half-life of 7 days. Olsalazine-S accumulates to steady state within 2 to 3 weeks.
Patients on daily doses of 1.0 g olsalazine for 2 to 4 years show a stable plasma concentration of olsalazine-S (3.3 to 12.4 µmol/L). Olsalazine-S is more than 99% bound to plasma proteins. Its long half-life is mainly due to slow dissociation from the protein binding site. Less than 1% of both olsalazine and olsalazine-S appears undissociated in plasma.
5-aminosalicylic acid (5-ASA): Serum concentrations of 5-ASA are detected after 4 to 8 hours. The peak levels of 5-ASA after an oral dose of 1.0 g olsalazine are low (i.e., 0 to 4.3 µmol/L). Of the total 5-ASA found in the urine, more than 90% is in the form of N-acetyl-5-ASA (Ac-5-ASA). Only small amounts of 5-ASA are detected.
N-acetyl-5-ASA (Ac-5-ASA), the major metabolite of 5-ASA found in plasma and urine, is acetylated (deactivated) in at least two sites, the colonic epithelium and the liver. Ac-5-ASA is found in the serum, with peak values of 1.7 to 8.7 µmol/L after a single 1.0 g dose. Approximately 20% of the total 5-ASA is recovered in the urine, where it is found almost exclusively as Ac-5-ASA. The remaining 5-ASA is partially acetylated and is excreted in the feces. From fecal dialysis, the concentration of 5-ASA in the colon following olsalazine has been calculated to be 18 to 49 mmol/L. No accumulation of 5-ASA or Ac-5-ASA in plasma has been detected. 5-ASA and Ac-5-ASA are 74 and 81%, respectively, bound to plasma proteins.
ANIMAL TOXICOLOGY
Preclinical subacute and chronic toxicity studies in rats have shown the kidney to be the major target organ of olsalazine toxicity. At an oral daily dose of 400 mg/kg or higher, olsalazine treatment produced nephritis and tubular necrosis in a 4-week study; interstitial nephritis and tubular calcinosis in a 6-month study, and renal fibrosis, mineralization, and transitional cell hyperplasia in a 1-year study.
Clinical Studies
Two controlled studies have demonstrated the efficacy of olsalazine as maintenance therapy in patients with ulcerative colitis. In the first, ulcerative colitis patients in remission were randomized to olsalazine 500 mg twice daily or placebo, and relapse rates for a six month period of time were compared. For the 52 patients randomized to olsalazine, 12 relapses occurred, while for the 49 placebo patients, 22 relapses occurred. This difference in relapse rates was significant (p<0.02).
In the second study, 164 ulcerative colitis patients in remission were randomized to olsalazine 500 mg twice daily or sulfasalazine 1 gram twice daily, and relapse rates were compared after six months. The relapse rate for olsalazine was 19.5% while that for sulfasalazine was 12.2%, a non-significant difference.
Indications and Usage for Dipentum
DIPENTUM is indicated for the maintenance of remission of ulcerative colitis in adult patients who are intolerant of sulfasalazine.
Contraindications
DIPENTUM is contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates or their metabolites, or to any of the excipients in DIPENTUM.
Warnings
Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure have been reported in patients given products that contain mesalamine or are converted to mesalamine. In animal studies, the kidney was the principal organ of mesalamine toxicity.
Evaluate the risks and benefits of using DIPENTUM in patients with known renal impairment or a history of renal disease or taking concomitant nephrotoxic drugs. Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on DIPENTUM therapy. Discontinue DIPENTUM if renal function deteriorates while on therapy.
Mesalamine-Induced Acute Intolerance Syndrome
Olsalazine is converted to mesalamine, which has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with DIPENTUM.
Hypersensitivity Reactions
Some patients who have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to DIPENTUM or to other compounds that contain or are converted to mesalamine. Mesalamine‑induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis, and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue DIPENTUM if an alternative etiology for the signs and symptoms cannot be established.
Precautions
Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Because olsalazine is converted to mesalamine, evaluate the risks and benefits of using DIPENTUM in patients with known liver impairment.
Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of mesalamine, the active moiety in DIPTENTUM (see ADVERSE REACTIONS). Discontinue DIPENTUM at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
Photosensitivity
Patients with pre‑existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, the active moiety in DIPENTUM, including stones with 100% mesalamine content. Mesalamine‑containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment.
Interference with Laboratory Tests
Use of DIPENTUM, which is converted to mesalamine, may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and mesalamine’s main metabolite, N‑acetyl‑5‑aminosalicylic acid (N‑Ac‑5‑ASA). Consider an alternative, selective assay for normetanephrine.
Information for Patients
Patients should be instructed to take DIPENTUM with food. The drug should be taken in evenly divided doses. Patients should be informed that about 17% of subjects receiving DIPENTUM during clinical studies reported diarrhea sometime during therapy. If diarrhea occurs, patients should contact their physician.
Drug Interactions
Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non‑steroidal anti‑inflammatory drugs (NSAIDs), may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions.
Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6‑mercaptopurine and/or any other drugs known to cause myelotoxicity (e.g., thioguanine) may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of DIPENTUM and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
Low Molecular Weight Heparins or Heparinoids
The co-administration of salicylates and low molecular weight heparins or heparinoids may result in an increased risk of bleeding (i.e., hematomas) following neuraxial anesthesia. Salicylates should be discontinued prior to the initiation of a low molecular weight heparin or heparinoid. If this is not possible, it is recommended to monitor patients closely for bleeding.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two year oral rat carcinogenicity study, olsalazine was tested in male and female Wistar rats at daily doses of 200, 400, and 800 mg/kg/day (approximately 10 to 40 times the human maintenance dose, based on a patient weight of 50 kg and a human dose of 1 g). Urinary bladder transitional cell carcinomas were found in three male rats (6%, p=0.022, exact trend test) receiving 40 times the human dose and were not found in untreated male controls. In the same study, urinary bladder transitional cell carcinoma and papilloma occurred in 2 untreated control female rats (2%). No such tumors were found in any of the female rats treated at doses up to 40 times the human dose.
In an eighteen month oral mouse carcinogenicity study, olsalazine was tested in male and female CD-1 mice at daily doses of 500, 1000, and 2000 mg/kg/day (approximately 25 to 100 times the human maintenance dose). Liver hemangiosarcomata were found in two male mice (4%) receiving olsalazine at 100 times the human dose, while no such tumor occurred in the other treated male mice groups or any of the treated female mice. The observed incidence of this tumor is within the 4% incidence in historical controls.
Olsalazine was not mutagenic in in vitro Ames tests, mouse lymphoma cell mutation assays, human lymphocyte chromosomal aberration tests, or the in vivo rat bone marrow cell chromosomal aberration test.
Olsalazine in a dose range of 100 to 400 mg/kg/day (approximately 5 to 20 times the human maintenance dose) did not influence the fertility of male or female rats. The oligospermia and infertility in men associated with sulfasalazine have not been reported with olsalazine.
Pregnancy
Teratogenic Effects
Olsalazine has been shown to produce fetal developmental toxicity as indicated by reduced fetal weights, retarded ossifications, and immaturity of the fetal visceral organs when given during organogenesis to pregnant rats in doses 5 to 20 times the human dose (100 to 400 mg/kg).
There are no adequate and well-controlled studies in pregnant women. Olsalazine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Small amounts of the active metabolite of olsalazine (5-ASA) may pass into breast milk. Harmful infant effects (diarrhea) have been reported when 5-ASA was used during breastfeeding. Unless the benefit of the treatment outweighs the risks, olsalazine should not be taken by breast-feeding women, or patients should be advised to discontinue breastfeeding if using olsalazine.
Oral administration of olsalazine to lactating rats in doses 5 to 20 times the human dose produced growth retardation in their pups.
Geriatric Use
Clinical studies of DIPENTUM did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia and pancytopenia) in patients receiving mesalamine-containing products such as DIPENTUM who were 65 years or older compared to younger patients, which may also be associated with ulcerative colitis, use of interacting drugs, or reduced renal function.
Consider monitoring of complete blood cell counts and platelet counts in elderly patients during treatment with DIPENTUM, especially if used concomitantly with anticoagulants. In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concurrent disease or other drug therapy in elderly patients when prescribing DIPENTUM.
Renal
Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions to DIPENTUM, which is converted to mesalamine, may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on DIPENTUM therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue DIPENTUM if renal function deteriorates while on therapy.
Adverse Reactions/Side Effects
Olsalazine has been evaluated in ulcerative colitis patients in remission, as well as those with acute disease. Both sulfasalazine-tolerant and intolerant patients have been studied in controlled clinical trials. Overall, 10.4% of patients discontinued olsalazine because of an adverse experience compared with 6.7% of placebo patients. The most commonly reported adverse reactions leading to treatment withdrawal were diarrhea or loose stools (olsalazine 5.9%; placebo 4.8%), abdominal pain, and rash or itching (slightly more than 1% of patients receiving olsalazine).
Diarrhea
Overall, approximately 17% of subjects receiving olsalazine in clinical studies reported diarrhea sometime during therapy. This diarrhea resulted in withdrawal of treatment in 6% of patients. This diarrhea appears to be dose related, although it may be difficult to distinguish from the underlying symptoms of the disease.
Other adverse reactions to olsalazine leading to withdrawal occurred in fewer than 1% of patients (Table 1).
|
Olsalazine
|
Placebo
|
|
|
Diarrhea/Loose Stools |
26 (5.9%) |
10 (4.8%) |
|
Nausea |
3 |
2 |
|
Abdominal Pain |
5 (1.1%) |
0 |
|
Rash/Itching |
5 (1.1%) |
0 |
|
Headache |
3 |
0 |
|
Heartburn |
2 |
0 |
|
Rectal Bleeding |
1 |
0 |
|
Insomnia |
1 |
0 |
|
Dizziness |
1 |
0 |
|
Anorexia |
1 |
0 |
|
Light Headedness |
1 |
0 |
|
Depression |
1 |
0 |
|
Miscellaneous |
4 (0.9%) |
3 (1.4%) |
|
Total Number of Patients Withdrawn |
46 (10.4%) |
14 (6.7%) |
For those controlled studies, the comparative incidences of adverse reactions reported in 1% or more patients treated with olsalazine or placebo are provided in Table 2.
| Adverse Event | Olsalazine
(N = 441) % | Placebo
(N = 208) % |
|---|---|---|
|
Gastrointestinal Disorders |
||
|
Diarrhea |
11.1 |
6.7 |
|
Abdominal Pain/Cramps |
10.1 |
7.2 |
|
Nausea |
5.0 |
3.9 |
|
Dyspepsia |
4.0 |
4.3 |
|
Bloating |
1.5 |
1.4 |
|
Vomiting |
1.0 |
- |
|
Stomatitis |
1.0 |
- |
|
Increased Blood in Stool |
- |
3.4 |
|
Metabolism and Nutrition Disorders |
||
|
Anorexia |
1.3 |
1.9 |
|
Nervous System Disorders |
||
|
Headache |
5.0 |
4.8 |
|
Insomnia |
- |
2.4 |
|
General Disorders and Administration Site Conditions |
||
|
Fatigue/Drowsiness/Lethargy |
1.8 |
2.9 |
|
Psychiatric Disorders |
||
|
Depression |
1.5 |
- |
|
Ear and Labyrinth Disorders |
||
|
Vertigo/Dizziness |
1.0 |
- |
|
Skin and Subcutaneous Tissue Disorders |
||
|
Rash |
2.3 |
1.4 |
|
Itching |
1.3 |
- |
|
Musculoskeletal and Connective Tissue Disorders |
||
|
Arthralgia/Joint Pain |
4.0 |
2.9 |
|
Infections and Infestations |
||
|
Upper Respiratory Infection |
1.5 |
- |
Over 2,500 patients have been treated with olsalazine in various controlled and uncontrolled clinical studies. In these as well as in post-marketing experience, olsalazine was administered mainly to patients intolerant to sulfasalazine. There have been rare reports of the following adverse effects in patients receiving olsalazine. These were often difficult to distinguish from possible symptoms of the underlying disease or from the effects of prior and/or concomitant therapy. A causal relationship to the drug has not been demonstrated for some of these reactions.
Blood and Lymphatic System Disorders: Anemia, Eosinophilia, Hemolytic anemia, Interstitial pulmonary disease, Leukopenia, Lymphopenia, Neutropenia, Reticulocytosis, Thrombocytopenia
Cardiac Disorders: Chest pains, Heart block second degree, Myocarditis, Palpitations, Pericarditis, Peripheral edema, Shortness of breath, Tachycardia
A patient who developed thyroid disease 9 days after starting DIPENTUM was given propranolol and radioactive iodine and subsequently developed shortness of breath and nausea. The patient died 5 days later with signs and symptoms of acute diffuse myocarditis.
Ear and Labyrinth Disorders: Tinnitus
Eye Disorders: Dry eyes, Vision blurred, Watery eyes
Gastrointestinal Disorders: Abdominal pain (upper), Diarrhea with dehydration, Dry mouth, Epigastric discomfort, Flare in symptoms, Flatulence, Increased blood in stool, Pancreatitis, Rectal bleeding, Rectal discomfort
In a double-blind, placebo-controlled study, increased frequency and severity of diarrhea were reported in patients randomized to olsalazine 500 mg B.I.D. with concomitant pelvic radiation.
Rare cases of granulomatous hepatitis and nonspecific, reactive hepatitis have been reported in patients receiving olsalazine. Additionally, a patient developed mild cholestatic hepatitis during treatment with sulfasalazine and experienced the same symptoms two weeks later after the treatment was changed to olsalazine. Withdrawal of olsalazine led to complete recovery in these cases.
General Disorders and Administration Site Conditions: Fever chills, Hot flashes, Irritability, Rigors
Immune System Disorders: Bronchospasm, Erythema nodosum
Laboratory: ALT (SGPT) or AST (SGOT) elevated beyond the normal range.
Musculoskeletal and Connective Tissue Disorders: Muscle cramps
Nervous System Disorders: Insomnia, Paraesthesia, Tremors
Psychiatric Disorders: Mood swings
Renal and Urinary Disorders: Dysuria, Hematuria, Interstitial nephritis, Nephrotic syndrome, Proteinuria, Urinary frequency
Reproductive System and Breast Disorders: Impotence, Menorrhagia
Skin and Subcutaneous Tissue Disorders: Alopecia, Erythema, Photosensitivity reaction
Vascular Disorders: Hypertension, Orthostatic hypotension
Postmarketing
The following events have been identified during post-approval use of products that contain (or are metabolized to) mesalamine in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of seriousness, frequency of reporting, or potential causal connection to mesalamine:
Blood and Lymphatic System Disorders: Aplastic anemia, Pancytopenia
General Disorders and Administration Site Conditions: Pyrexia
Hepatobiliary Disorders: Hepatic enzyme increased, Hepatitis, Increased bilirubin
Reports of hepatotoxicity, including elevated liver function tests (SGOT/AST, SGPT/ALT, GGT, LDH, alkaline phosphatase, bilirubin), jaundice, cholestatic jaundice, cirrhosis, and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. One case of Kawasaki-like syndrome, which included hepatic function changes, was also reported.
Musculoskeletal and Connective Tissue Disorders: Myalgia
Respiratory, Thoracic and Mediastinal Disorders: Dyspnea, Interstitial lung disease, pleurisy/pleuritis
Skin and Subcutaneous Tissue Disorders: Angioneurotic edema, SJS/TEN, DRESS, and AGEP
Nervous System Disorders: Paresthesia, Peripheral neuropathy
Renal and Urinary Disorders: Interstitial nephritis, nephrolithiasis
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-888-380-3276 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
DIPENTUM is an aminosalicylate, and symptoms of salicylate toxicity include: nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) damage. There is no specific antidote for olsalazine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage and may include gastrointestinal tract decontamination to prevent of further absorption. Proper medical care should be sought immediately with appropriate supportive care, including the possible use of emesis, cathartics, and activated charcoal to prevent further absorption. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function.
Dipentum Dosage and Administration
The recommended dosage in adults for maintenance of remission of ulcerative colitis is 500 mg twice daily.
Drink an adequate amount of fluids during treatment.
How is Dipentum supplied
Beige colored capsules, containing 250 mg olsalazine sodium imprinted with “DIPENTUM® 250 mg” on the capsule shell, available as:
Bottles of 100’s NDC 0037-6860-10
Store at 20-25°C (77°F). Excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Manufactured for:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
Manufactured by:
Societal CDMO Gainesville, LLC
Gainesville, GA 30504, USA
© 2022 Viatris Inc.
DIPENTUM is a registered trademark of Alaven Pharmaceutical LLC, a Viatris Company.
Rev. 11/2022
IN-686010-06
Material Code: 6003064-04
PRINCIPAL DISPLAY PANEL – 250 mg
NDC 0037-6860-10
Dipentum®
(olsalazine sodium)
capsule, gelatin coated
250 mg
Rx only
100 Capsules
USUAL DOSAGE: See package circular
for complete product information.
PHARMACIST: Dispense in a
well-closed container.
Store at 25°C (77°F); excursions
15°-30°C (59°-86°F).
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
Manufactured by:
Societal CDMO Gainesville, LLC
Gainesville, GA 30504, USA
©2022 Viatris Inc.
DIPENTUM is a registered trademark
of Alaven Pharmaceutical LLC,
a Viatris Company.
LB-686010-04
6003065-03
| DIPENTUM
olsalazine sodium capsule, gelatin coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Meda Pharmaceuticals Inc. (051229602) |