Drug Detail:Duoneb (Albuterol and ipratropium (inhalation) [ al-bue-ter-ol-and-ip-ra-tro-pee-um ])
Drug Class: Bronchodilator combinations
Related/similar drugs
Symbicort, Breo Ellipta, Ventolin, Spiriva, Ventolin HFA, Anoro ElliptaDuoNeb - Clinical Pharmacology
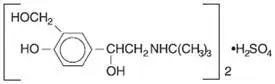
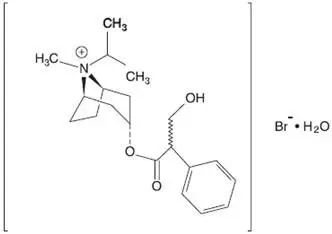
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is a combination of the β2-adrenergic bronchodilator, albuterol sulfate, and the anticholinergic bronchodilator, ipratropium bromide.
Precautions
Adverse Reactions/Side Effects
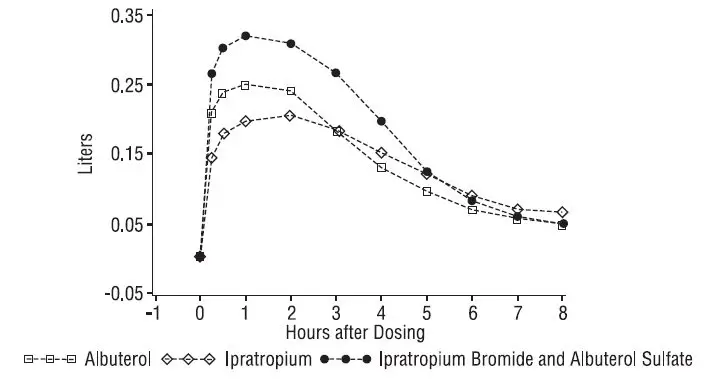
Adverse reaction information concerning Ipratropium Bromide and Albuterol Sulfate Inhalation Solution was derived from the 12-week controlled clinical trial.
| Body System COSTART Term | Albuterol n (%) | Ipratropium n (%) | Ipratropium and Albuterol n (%) |
|---|---|---|---|
| NUMBER OF PATIENTS | 761 | 754 | 765 |
| N (%) Patients with AE | 327 (43.0) | 329 (43.6) | 367 (48.0) |
| BODY AS A WHOLE | |||
| Pain | 8 (1.1) | 4 (0.5) | 10 (1.3) |
| Pain chest | 11 (1.4) | 14 (1.9) | 20 (2.6) |
| DIGESTIVE | |||
| Diarrhea | 5 (0.7) | 9 (1.2) | 14 (1.8) |
| Dyspepsia | 7 (0.9) | 8 (1.1) | 10 (1.3) |
| Nausea | 7 (0.9) | 6 (0.8) | 11 (1.4) |
| MUSCULO-SKELETAL | |||
| Cramps leg | 8 (1.1) | 6 (0.8) | 11 (1.4) |
| RESPIRATORY | |||
| Bronchitis | 11 (1.4) | 13 (1.7) | 13 (1.7) |
| Lung Disease | 36 (4.7) | 34 (4.5) | 49 (6.4) |
| Pharyngitis | 27 (3.5) | 27 (3.6) | 34 (4.4) |
| Pneumonia | 7 (0.9) | 8 (1.1) | 10 (1.3) |
| UROGENITAL | |||
| Infection urinary tract | 3 (0.4) | 9 (1.2) | 12 (1.6) |
Additional adverse reactions reported in more than 1% of patients treated with Ipratropium Bromide and Albuterol Sulfate Inhalation Solution included constipation and voice alterations.
In the clinical trial, there was a 0.3% incidence of possible allergic-type reactions, including skin rash, pruritus, and urticaria.
Additional information derived from the published literature on the use of albuterol sulfate and ipratropium bromide singly or in combination includes precipitation or worsening of narrow-angle glaucoma, acute eye pain, blurred vision, mydriasis, paradoxical bronchospasm, wheezing, exacerbation of COPD symptoms, drowsiness, aching, flushing, upper respiratory tract infection, palpitations, taste perversion, elevated heart rate, sinusitis, back pain, sore throat and metabolic acidosis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
DuoNeb Dosage and Administration
The recommended dose of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is one 3 mL vial administered 4 times per day via nebulization with up to 2 additional 3 mL doses allowed per day, if needed. Safety and efficacy of additional doses or increased frequency of administration of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution beyond these guidelines has not been studied and the safety and efficacy of extra doses of albuterol sulfate or ipratropium bromide in addition to the recommended doses of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution have not been studied.
The use of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution can be continued as medically indicated to control recurring bouts of bronchospasm. If a previously effective regimen fails to provide the usual relief, medical advice should be sought immediately, as this is often a sign of worsening COPD, which would require reassessment of therapy.
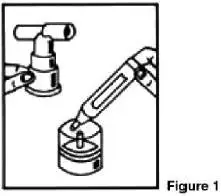
A Pari-LC-Plus™ nebulizer (with face mask or mouthpiece) connected to a PRONEB™ compressor was used to deliver Ipratropium Bromide and Albuterol Sulfate Inhalation Solution to each patient in one U.S. clinical study. The safety and efficacy of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution delivered by other nebulizers and compressors have not been established.
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be administered via jet nebulizer connected to an air compressor with an adequate air flow, equipped with a mouthpiece or suitable face mask.
| DUONEB
ipratropium bromide and albuterol sulfate solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Ritedose Pharmaceuticals, LLC (968062294) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The Ritedose Corporation | 837769546 | MANUFACTURE(76204-600) , LABEL(76204-600) , PACK(76204-600) , ANALYSIS(76204-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lusochimica SPA | 428179048 | API MANUFACTURE(76204-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TEVA Pharmaceuticals | 428573708 | API MANUFACTURE(76204-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Quality Chemical Laboratories | 071344167 | ANALYSIS(76204-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PPD Development | 838082055 | ANALYSIS(76204-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Polymer Solutions Inc. | 197129828 | ANALYSIS(76204-600) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NSF-Pharmalytica | 030955129 | ANALYSIS(76204-600) | |