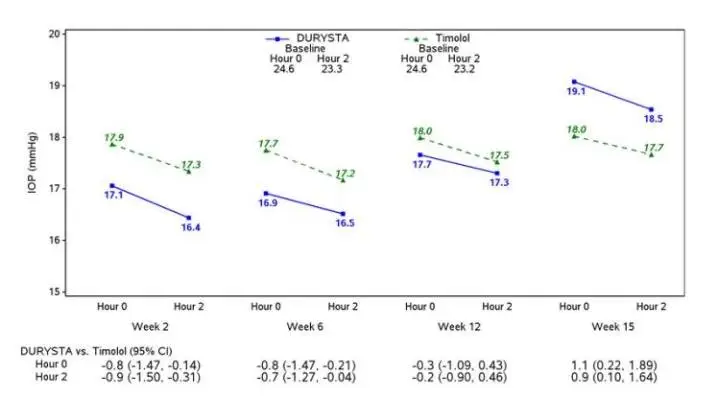
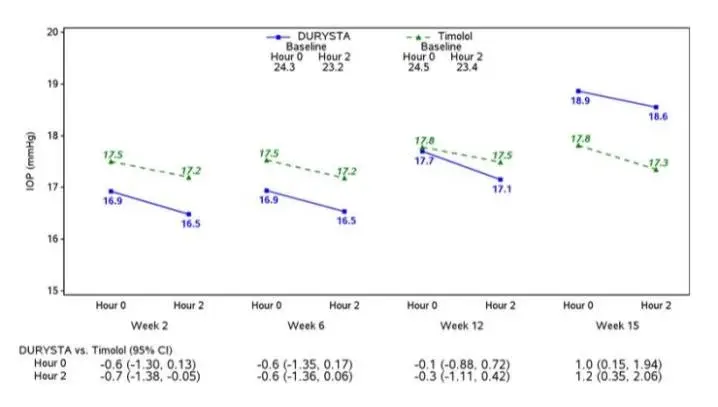
Drug Detail:Durysta (Bimatoprost ophthalmic implant [ bih-mat-o-prost ])
Drug Class: Ophthalmic glaucoma agents
Highlights of Prescribing Information
DURYSTATM (bimatoprost intracameral implant), for intracameral administration
Initial U.S. Approval: 2001
Indications and Usage for Durysta
DURYSTATM is a prostaglandin analog indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT). (1)
Durysta Dosage and Administration
- For ophthalmic intracameral administration. (2.1)
- The intracameral administration should be carried out under standard aseptic conditions. (2.2)
Dosage Forms and Strengths
Intracameral implant containing bimatoprost 10 mcg, in a drug delivery system. (3)
Contraindications
- Ocular or periocular infections (4.1)
- Corneal endothelial cell dystrophy (4.2)
- Prior corneal transplantation (4.3)
- Absent or ruptured posterior lens capsule (4.4)
- Hypersensitivity (4.5)
Warnings and Precautions
-
Endothelial cell loss: Due to possible corneal endothelial cell loss, administration of DURYSTA should be limited to a single implant per eye without retreatment. (5.1)
-
Corneal Adverse Reactions: DURYSTA has been associated with corneal adverse reactions and risks are increased with multiple implants. Use caution in patients with limited corneal endothelial cell reserve. (5.1)
- Iridocorneal Angle: DURYSTA should be used with caution in patients with narrow angles or anatomical angle obstruction. (5.2)
Adverse Reactions/Side Effects
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5-10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2020
Full Prescribing Information
1. Indications and Usage for Durysta
DURYSTATM (bimatoprost intracameral implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
2. Durysta Dosage and Administration
2.1 General Information
DURYSTA is an ophthalmic drug delivery system for a single intracameral administration of a biodegradable implant. DURYSTA should not be readministered to an eye that received a prior DURYSTA.
2.2 Administration
The intracameral injection procedure must be performed under magnification that allows clear visualization of the anterior chamber structures and should be carried out using standard aseptic conditions for intracameral procedures, with the patient’s head in a stabilized position. The eye should not be dilated prior to the procedure.
Remove the foil pouch from the carton and examine for damage. Then, open the foil pouch over a sterile field and gently drop the applicator on a sterile tray. Once the foil pouch is opened, use promptly.
Figure 1
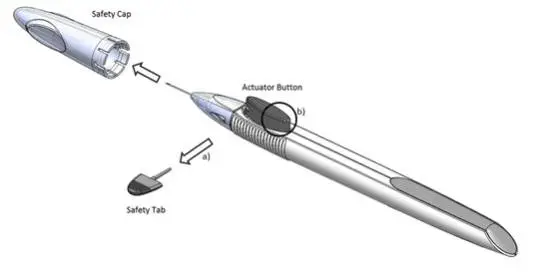
Perform a detailed visual inspection of the applicator, including ensuring that the actuator button has not been depressed, and the safety tab is in place. Carefully remove the plastic safety cap taking care to avoid contacting the needle tip. Inspect the needle tip for damage under magnification prior to use; the implant retention plug may be visible in the bevel and should not be removed.
Prior to use, remove the safety tab by pulling it out perpendicular to the long axis of the applicator (refer to Figure 1a above). Do not twist or bend the tab.
Stabilize the eye as the needle is advanced through the cornea. Enter the anterior chamber with the needle bevel visible through clear cornea. Enter parallel to the iris plane, adjacent to the limbus through clear cornea in the superotemporal quadrant.
The needle should be inserted approximately 2 bevel lengths with the bevel completely within the anterior chamber; avoid positioning the needle bevel directly over the pupil. Ensure the needle is not bent before depressing the actuator button. See Figure 2.
Figure 2
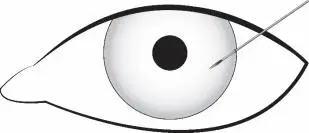
Depress the back half of the actuator button (refer to Figure 1b above) firmly until an audible and/or palpable click is noted.
Following the release of the implant, remove the needle via the same track in which it was inserted and tamponade the opening. The implant should not be left in the corneal injection track.
Check the injection site for leaks; make sure that it is self-sealing and the anterior chamber is formed.
After injection, do not recap the needle. Dispose of the used applicator in a sharps disposal container and in accordance with local requirements.
Instruct the patient to remain upright for at least 1 hour after the procedure so the implant can settle.
Some degree of eye redness and discomfort is expected following administration. However, it is recommended to instruct patients that if the eye becomes progressively red, sensitive to light, painful, or develops a change in vision, they should immediately contact the physician.
3. Dosage Forms and Strengths
Intracameral implant containing 10 mcg of bimatoprost in a drug delivery system.
4. Contraindications
4.1 Ocular or Periocular Infections
DURYSTA is contraindicated in patients with active or suspected ocular or periocular infections.
4.2 Corneal Endothelial Cell Dystrophy
DURYSTA is contraindicated in patients with corneal endothelial cell dystrophy (e.g., Fuchs’ Dystrophy) [see Warnings and Precautions (5.1)].
4.3 Prior Corneal Transplantation
DURYSTA is contraindicated in patients with prior corneal transplantation, or endothelial cell transplants [e.g., Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK)].
4.4 Absent or Ruptured Posterior Lens Capsule
DURYSTA is contraindicated in patients whose posterior lens capsule is absent or ruptured, due to the risk of implant migration into the posterior segment. Laser posterior capsulotomy in pseudophakic patients is not a contraindication for DURYSTA use if the intraocular lens fully covers the opening in the posterior capsule.
5. Warnings and Precautions
5.1 Corneal Adverse Reactions
The presence of DURYSTA implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA in patients with limited corneal endothelial cell reserve.
5.2 Iridocorneal Angle
Following administration with DURYSTA, the intracameral implant is intended to settle within the inferior angle. DURYSTA should be used with caution in patients with narrow iridocorneal angles (Shaffer grade ˂ 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
5.3 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA intracameral implant. DURYSTA should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
5.4 Intraocular Inflammation
Prostaglandin analogs, including DURYSTA, have been reported to cause intraocular inflammation. DURYSTA should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
5.5 Pigmentation
Ophthalmic bimatoprost, including DURYSTA intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. While treatment with DURYSTA can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in labeling:
- Implant migration [see Contraindications (4.4)]
- Hypersensitivity [see Contraindications (4.5)]
- Corneal adverse reactions [see Warnings and Precautions (5.1)]
- Macular edema [see Warnings and Precautions (5.3)]
- Intraocular inflammation [see Warnings and Precautions (5.4)]
- Pigmentation [see Warnings and Precautions (5.5)]
- Endophthalmitis [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common ocular adverse reaction observed in two randomized, active-controlled clinical trials with DURYSTA in patients with OAG or OHT was conjunctival hyperemia, which was reported in 27% of patients. Other common ocular adverse reactions reported in 5-10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, and iritis. Ocular adverse reactions occurring in 1-5% of patients were anterior chamber cell, lacrimation increased, corneal edema, aqueous humor leakage, iris adhesions, ocular discomfort, corneal touch, iris hyperpigmentation, anterior chamber flare, anterior chamber inflammation, and macular edema. The following additional adverse drug reactions occurred in less than 1% of patients: hyphema, iridocyclitis, uveitis, corneal opacity, product administered at inappropriate site, corneal decompensation, cystoid macular edema, and drug hypersensitivity.
The most common nonocular adverse reaction was headache, which was observed in 5% of patients.
| DURYSTA
bimatoprost implant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |




![The chemical name for bimatoprost is (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 415.57. Its molecular formula is C25H37NO4.](https://cdn.themeditary.com/images/2023/09/03/durysta-03.webp)