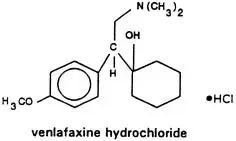
Drug Detail:Venlafaxine (Venlafaxine [ ven-la-fax-een ])
Drug Class: Serotonin-norepinephrine reuptake inhibitors
Effexor - Clinical Pharmacology
Related/similar drugs
Rexulti, Trintellix, sertraline, trazodone, fluoxetine, Lexapro, venlafaxineClinical Studies
Indications and Usage for Effexor
Effexor (venlafaxine hydrochloride) is indicated for the treatment of major depressive disorder.
Contraindications
Hypersensitivity to venlafaxine hydrochloride or to any excipients in the formulation.
Warnings
Clinical Worsening and Suicide Risk
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
| Increases Compared to Placebo | |
| <18 | 14 additional cases |
| 18-24 | 5 additional cases |
| Decreases Compared to Placebo | |
| 25-64 | 1 fewer case |
| ≥65 | 6 fewer cases |
Precautions
General
Anxiety and Insomnia
| Symptom | Venlafaxine n = 1033 | Placebo n = 609 |
| Anxiety | 6% | 3% |
| Nervousness | 13% | 6% |
| Insomnia | 18% | 10% |
Information for Patients
Drug Interactions
As with all drugs, the potential for interaction by a variety of mechanisms is a possibility.
Labor and Delivery
The effect of Effexor® (venlafaxine hydrochloride) on labor and delivery in humans is unknown.
Adverse Reactions/Side Effects
Associated with Discontinuation of Treatment
| * Percentages based on the number of males. | ||
| CNS | Venlafaxine | Placebo |
| Somnolence | 3% | 1% |
| Insomnia | 3% | 1% |
| Dizziness | 3% | — |
| Nervousness | 2% | — |
| Dry mouth | 2% | — |
| Anxiety | 2% | 1% |
| Gastrointestinal | ||
| Nausea | 6% | 1% |
| Urogenital | ||
| Abnormal ejaculation* | 3% | — |
| Other | ||
| Headache | 3% | 1% |
| Asthenia | 2% | — |
| Sweating | 2% | — |
Incidence in Controlled Trials
Adverse Events Occurring at an Incidence of 1% or More Among Effexor-Treated Patients
| Body System | Preferred Term | Effexor
(n=1033) | Placebo
(n=609) |
|---|---|---|---|
| 1 Events reported by at least 1% of patients treated with Effexor (venlafaxine hydrochloride) are included, and are rounded to the nearest %. Events for which the Effexor incidence was equal to or less than placebo are not listed in the table, but included the following: abdominal pain, pain, back pain, flu syndrome, fever, palpitation, increased appetite, myalgia, arthralgia, amnesia, hypesthesia, rhinitis, pharyngitis, sinusitis, cough increased, and dysmenorrhea3. | |||
| Body as a Whole | Headache | 25% | 24% |
| Asthenia | 12% | 6% | |
| Infection | 6% | 5% | |
| Chills | 3% | — | |
| Chest pain | 2% | 1% | |
| Trauma | 2% | 1% | |
| Cardiovascular | Vasodilatation | 4% | 3% |
| Increased blood pressure/hypertension | 2% | — | |
| Tachycardia | 2% | — | |
| Postural hypotension | 1% | — | |
| Dermatological | Sweating | 12% | 3% |
| Rash | 3% | 2% | |
| Pruritus | 1% | — | |
| Gastrointestinal | Nausea | 37% | 11% |
| Constipation | 15% | 7% | |
| Anorexia | 11% | 2% | |
| Diarrhea | 8% | 7% | |
| Vomiting | 6% | 2% | |
| Dyspepsia | 5% | 4% | |
| Flatulence | 3% | 2% | |
| Metabolic | Weight loss | 1% | — |
| Nervous System | Somnolence | 23% | 9% |
| Dry mouth | 22% | 11% | |
| Dizziness | 19% | 7% | |
| Insomnia | 18% | 10% | |
| Nervousness | 13% | 6% | |
| Anxiety | 6% | 3% | |
| Tremor | 5% | 1% | |
| Abnormal dreams | 4% | 3% | |
| Hypertonia | 3% | 2% | |
| Paresthesia | 3% | 2% | |
| Libido decreased | 2% | — | |
| Agitation | 2% | — | |
| Confusion | 2% | 1% | |
| Thinking abnormal | 2% | 1% | |
| Depersonalization | 1% | — | |
| Depression | 1% | — | |
| Urinary retention | 1% | — | |
| Twitching | 1% | — | |
| Respiration | Yawn | 3% | — |
| Special Senses | Blurred vision | 6% | 2% |
| Taste perversion | 2% | — | |
| Tinnitus | 2% | — | |
| Mydriasis | 2% | — | |
| Urogenital System | Abnormal ejaculation/ orgasm | 12%2 | —2 |
| Impotence | 6%2 | —2 | |
| Urinary frequency | 3% | 2% | |
| Urination impaired | 2% | — | |
| Orgasm disturbance | 2%3 | —3 | |
Dose Dependency of Adverse Events
| Effexor (mg/day) | ||||
|---|---|---|---|---|
| Body System/ Preferred Term | ||||
| Placebo (n=92) | 75 (n=89) | 225 (n=89) | 375 (n=88) | |
| Body as a Whole | ||||
| Abdominal pain | 3.3% | 3.4% | 2.2% | 8.0% |
| Asthenia | 3.3% | 16.9% | 14.6% | 14.8% |
| Chills | 1.1% | 2.2% | 5.6% | 6.8% |
| Infection | 2.2% | 2.2% | 5.6% | 2.3% |
| Cardiovascular System | ||||
| Hypertension | 1.1% | 1.1% | 2.2% | 4.5% |
| Vasodilatation | 0.0% | 4.5% | 5.6% | 2.3% |
| Digestive System | ||||
| Anorexia | 2.2% | 14.6% | 13.5% | 17.0% |
| Dyspepsia | 2.2% | 6.7% | 6.7% | 4.5% |
| Nausea | 14.1% | 32.6% | 38.2% | 58.0% |
| Vomiting | 1.1% | 7.9% | 3.4% | 6.8% |
| Nervous System | ||||
| Agitation | 0.0% | 1.1% | 2.2% | 4.5% |
| Anxiety | 4.3% | 11.2% | 4.5% | 2.3% |
| Dizziness | 4.3% | 19.1% | 22.5% | 23.9% |
| Insomnia | 9.8% | 22.5% | 20.2% | 13.6% |
| Libido decreased | 1.1% | 2.2% | 1.1% | 5.7% |
| Nervousness | 4.3% | 21.3% | 13.5% | 12.5% |
| Somnolence | 4.3% | 16.9% | 18.0% | 26.1% |
| Tremor | 0.0% | 1.1% | 2.2% | 10.2% |
| Respiratory System | ||||
| Yawn | 0.0% | 4.5% | 5.6% | 8.0% |
| Skin and Appendages | ||||
| Sweating | 5.4% | 6.7% | 12.4% | 19.3% |
| Special Senses | ||||
| Abnormality of accommodation | 0.0% | 9.1% | 7.9% | 5.6% |
| Urogenital System | ||||
| Abnormal ejaculation/orgasm | 0.0% | 4.5% | 2.2% | 12.5% |
| Impotence | 0.0% | 5.8% | 2.1% | 3.6% |
| (Number of men) | (n=63) | (n=52) | (n=48) | (n=56) |
Other Events Observed During the Premarketing Evaluation of Venlafaxine
Endocrine system—Rare: goiter, hyperthyroidism, hypothyroidism, thyroid nodule, thyroiditis.
Effexor Dosage and Administration
Special Populations
How is Effexor supplied
Effexor® (venlafaxine hydrochloride) Tablets are available as follows:
25 mg, peach, shield-shaped tablet with “25” and a “W” on one side and “701” on scored reverse side.
NDC 0008-0701-08, bottle of 60 tablets in unit of use package.
NDC 0008-0781-08, bottle of 60 tablets in unit of use package.
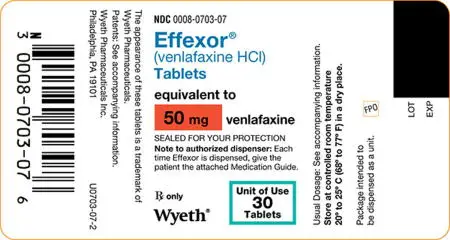
50 mg, peach, shield-shaped tablet with “50” and a “W” on one side and “703” on scored reverse side.
NDC 0008-0703-07, bottle of 30 tablets in unit of use package.
75 mg, peach, shield-shaped tablet with “75” and a “W” on one side and “704” on scored reverse side.
NDC 0008-0704-07, bottle of 30 tablets in unit of use package.
NDC 0008-0705-07, bottle of 20 tablets in unit of use package.
The appearance of these tablets is a trademark of Wyeth Pharmaceuticals.
Store at controlled room temperature 20° to 25°C (68° to 77°F) in a dry place.
Dispense in a well-closed container as defined in the USP.
The unit of use package is intended to be dispensed as a unit.
Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
|
|
|
|
|
|
|
|
|
|
|
|
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
 | This product's label may have been updated. For current package insert and further product information, please visit www.wyeth.com or call our medical communications department toll-free at 1-800-934-5556. |  |
| EFFEXOR
venlafaxine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| EFFEXOR
venlafaxine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| EFFEXOR
venlafaxine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| EFFEXOR
venlafaxine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| EFFEXOR
venlafaxine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Wyeth Pharmaceuticals Company (071170729) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wyeth Pharmaceuticals Company | 071170729 | MANUFACTURE, ANALYSIS | |